High-Yield Terms to Learn
Cobalamin Vitamin B12 dTMP synthesis A set of biochemical reactions that produce deoxythymidylate (dTMP), an essential constituent of DNA synthesis. The cycle depends on the conversion of dihydrofolate to tetrahydrofolate by dihydrofolate reductase (Figure 33-1) G-CSF Granulocyte colony-stimulating factor, a hematopoietic growth factor that regulates production and function of neutrophils GM-CSF Granulocyte-macrophage colony-stimulating factor, a hematopoietic growth factor that regulates production of granulocytes (basophils, eosinophils, and neutrophils), and other myeloid cells Hemochromatosis A condition of chronic excess total body iron caused either by an inherited abnormality of iron absorption or by frequent transfusions to treat certain types of hemolytic disorders (eg, thalassemia major) Megaloblastic anemia A deficiency in serum hemoglobin and erythrocytes in which the erythrocytes are abnormally large. Results from either folate or vitamin B12 deficiency anemia Microcytic anemia A deficiency in serum hemoglobin and erythrocytes in which the erythrocytes are abnormally small. Often caused by iron deficiency Neutropenia An abnormally low number of neutrophils in the blood; patients with neutropenia are susceptible to serious infection Pernicious anemia A form of megaloblastic anemia resulting from deficiency of intrinsic factor, a protein produced by gastric mucosal cells and required for intestinal absorption of vitamin B12 Thrombocytopenia An abnormally low number of platelets in the blood; patients with thrombocytopenia are susceptible to hemorrhage
Blood Cell Deficiencies
Iron and Vitamin Deficiency Anemias
Microcytic hypochromic anemia, caused by iron deficiency, is the most common type of anemia. Megaloblastic anemias are caused by a deficiency of vitamin B12 or folic acid, cofactors required for the normal maturation of red blood cells. Pernicious anemia, the most common type of vitamin B12 deficiency anemia, is caused by a defect in the synthesis of intrinsic factor, a protein required for efficient absorption of dietary vitamin B12, or by surgical removal of that part of the stomach that secretes intrinsic factor.
Other Blood Cell Deficiencies
Deficiency in the concentration of the various lineages of blood cells can be a manifestation of a disease or a side effect of radiation or cancer chemotherapy. Recombinant DNA-directed synthesis of hematopoietic growth factors now makes possible the treatment of more patients with deficiencies in erythrocytes, neutrophils, and platelets. Some of these growth factors also play an important role in hematopoietic stem cell transplantation.
Iron
Role of Iron
Iron is the essential metallic component of heme, the molecule responsible for the bulk of oxygen transport in the blood. Although most of the iron in the body is contained in hemoglobin, an important fraction is bound to transferrin, a transport protein, and ferritin, a storage protein. Deficiency of iron occurs most often in women because of menstrual blood loss and in vegetarians or malnourished persons because of inadequate dietary iron intake. Children and pregnant women have increased requirements for iron.
Regulation of Iron Stores
Although iron is an essential ion, excessive amounts are highly toxic. As a result, a complex system has evolved for the transport and storage of free iron (Figure 33-1). Since there is no mechanism for the efficient excretion of iron, regulation of body iron content occurs through modulation of intestinal absorption.
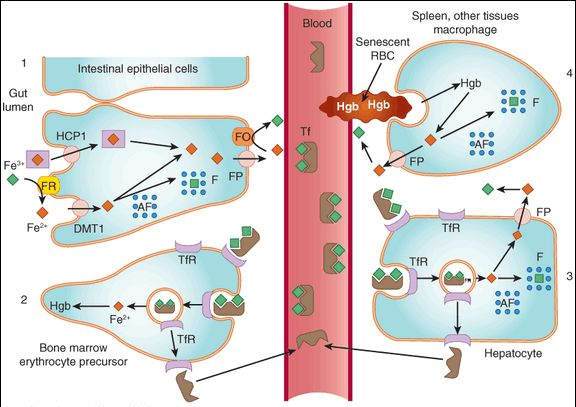
FIGURE 33-1
Absorption, transport, and storage of iron. Intestinal epithelial cells actively absorb inorganic iron via the divalent metal transporter (DMT1) and heme iron via the heme carrier protein 1 (HCP1) (1). Iron that is absorbed or released from absorbed heme iron is actively transported into the blood by ferroportin (FP) or complexed with apoferritin (AF) and stored as ferritin. In the blood, iron is transported by transferrin (Tf) to erythroid precursors in the bone marrow for synthesis of hemoglobin (Hb) (2) or to hepatocytes for storage as ferritin (3). The transferrin iron complex binds to transferrin receptors (TfR) in erythroid precursors and hepatocytes and is internalized. After release of the iron, the TfR-Tf complex is recycled to the plasma membrane and Tf is released. Macrophages that phagocytize senescent erythrocytes (RBC) reclaim the iron from the RBC hemoglobin and either export it or store it as ferritin (4). Hepatocytes use several mechanisms to take up iron and store the iron as ferritin. FO, ferroxidase; FP, ferroportin; FR, ferrireductase.
(Modified and reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 33-1.)
Absorption
Dietary iron in the form of heme and the ferrous ion (Fe2+) are taken up by a specialized divalent metal transporter 1 (DMT1) in intestinal epithelials cells. Intestinal cell iron is either stored as ferritin or the ferrous iron is transported across the basolateral membrane by ferroportin and then oxidized to ferric iron (Fe3+) by a ferroxidase (Figure 33-1).
Transport and Storage
Ferric iron is transported in a complex with transferrin. Excess iron is stored in the protein-bound form in gastrointestinal epithelial cells, macrophages, and hepatocytes, and, in cases of gross overload, in parenchymal cells of the skin, heart, and other organs.
Elimination
Minimal amounts of iron are lost from the body with sweat and saliva and in exfoliated skin and intestinal mucosal cells.
Clinical Use
Prevention or treatment of iron deficiency anemia is the only indication for iron administration. Iron deficiency can be diagnosed from red blood cell changes (microcytic cell size due to diminished hemoglobin content) and from measurements of serum and bone marrow iron stores. The disease is treated by dietary ferrous iron supplementation with ferrous sulfate, ferrous gluconate, or ferrous fumarate. In special cases, treatment is by parenteral administration of a colloid containing a core of iron oxyhydroxide surrounded by a core of carbohydrate. Parenteral iron preparations include iron dextran, sodium ferric gluconate complex, and iron sucrose. Iron should not be given in hemolytic anemia because iron stores are elevated, not depressed, in this type of anemia.
Toxicity of Iron (see also Chapter 57)
Signs and Symptoms
Acute iron intoxication is most common in children and usually occurs as a result of accidental ingestion of iron supplementation tablets. Depending on the dose of iron, necrotizing gastroenteritis, shock, metabolic acidosis, coma, and death may result. Chronic iron overload, known as hemochromatosis, damages the organs that store excess iron (heart, liver, pancreas). Hemochromatosis occurs most often in persons with an inherited abnormality of iron absorption and those who receive frequent transfusions for treatment of hemolytic disorders (eg, thalassemia major).
Treatment of Acute Iron Intoxication
Immediate treatment is necessary and usually consists of removal of unabsorbed tablets from the gut, correction of acid-base and electrolyte abnormalities, and parenteral administration of deferoxamine, which chelates circulating iron.
Treatment of Chronic Iron Toxicity
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree