Figure 1 Mechanisms of action for antibiotics.
Reproduced from Pratt C and Cornely K (2004) Essential Biochemistry. John Wiley and Sons: NY, USA.
Bacteria have developed many ways to resist inhibition or killing by antimicrobial agents, some of which are illustrated in Figure 2. Since antibiotics are produced by organisms in the environment, these resistance mechanisms have been evolving along with bacteria for millions of years. This evolution of resistance was greatly accelerated by the use of antibiotics in the treatment of human and animal infections in the past 60 years. Antibiotic resistance can be intrinsic to a particular group of bacteria or can be achieved through mutation or acquisition of antibiotic resistance genes from other organisms through conjugation, transformation, or transduction.
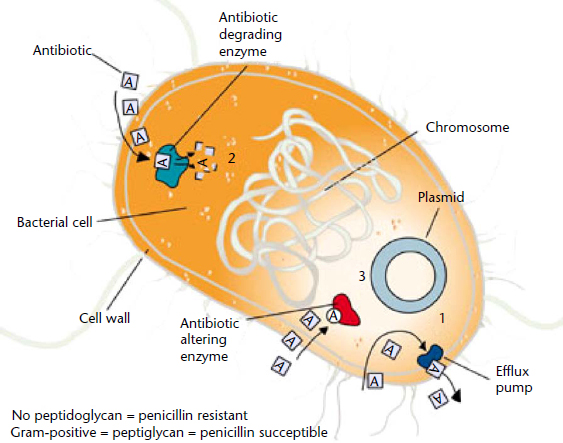
Figure 2 Mechanisms of antibiotic resistance.
Reproduced from Pratt C and Cornely K (2004) Essential Biochemistry. John Wiley and Sons: NY, USA.
The major mechanisms of antibiotic resistance in bacteria include:
Treatment of beta-hemolytic streptococcal (BHS) infections
Pharyngitis and skin infections such as impetigo are the most frequent conditions caused by GAS and are usually easily treatable. Proper diagnosis of GAS pharyngitis is important because while it is the most common cause of bacterial pharyngitis and requires antibiotic therapy, it is difficult to distinguish from viral pharyngitis, for which antibiotic treatment is neither effective nor appropriate. Rapid antigen detection testing RADT) is performed frequently, but throat culture is still the most sensitive and specific test for diagnosis of GAS infection (Bisno et al., 2002).
β-Lactams. Even after more than a half a century of penicillin use it is still the antibiotic of choice when treating BHS infections. There are no reports of penicillin- or β-lactam-resistant BHS. A recent population-based surveillance study by the CDC evaluating 6207 invasive GAS infections during 1999–2009 revealed no penicillin or cephalosporin resistance in isolates collected from seven ABC sites in the United States (Van Beneden et al., 2010). This is also the case for GBS and NABS.
Macrolides. For patients allergic to β-lactams, macrolides such as erythromycin are the antibiotic of choice. Unfortunately, resistance to macrolides has emerged worldwide in GAS, GBS and NABS. Macrolide resistance is mediated predominantly by the efflux gene mefA and the methylase genes erm(A) and erm(B) (Robinson et al., 2006). In a 10-year surveillance study by the CDC, erythromycin resistance in GAS averaged 10.1% (Van Beneden et al., 2010). The predominant erythromycin-resistant determinant found over the study period was erm(TR), a subclass of erm(A), accounting for 66% of the isolates. Erythromycin resistance rates were highly variable over the study period and also varied depending on the ABC site being studied.
Clindamycin, a lincosamide antibiotic, is indicated when the organism is macrolide-resistant. It is also used for serious invasive infections such as necrotising fasciitis and STSS IV clindamycin is recommended because it interferes with the toxin production, which leads to tissue destruction and organ failure. In the case of necrotising fasciitis, a broad-spectrum cephalosporin or metronidazole may also be used for coverage of anaerobic bacteria. Aggressive surgery is also needed to open infected areas and to remove necrotic tissue.
GBS are the leading cause of sepsis in newborns in the United States. Universal guidelines have been adopted to help prevent GBS disease in newborns in the United States (CDC, strep B prevention guidelines, 2010b, d). Screening of women for GBS (S. agalactiae) colonisation and intrapartum antibiotic therapy has dramatically decreased the number of cases of early onset GBS disease, however some women who test negative for GBS during weeks 35–37 may have undetected GBS in the birth canal during childbirth and pass this on to their infant. Therefore it is essential to monitor neonates closely for symptoms of GBS infection, which usually occur within the first 24 h.
Prevention of early onset GBS infection: It is recommended that colonised mothers be treated with penicillin G or ampicillin. If she is allergic to penicillins and does not have a history of anaphylaxis, angioedema, respiratory distress, or urticaria following treatment with a penicillin or a cephalosporin, she may receive cefazolin. Otherwise clindamycin is recommended as long as the organism is susceptible to both clindamycin and erythromycin. A special susceptibility test must be performed to determine whether the organism has inducible resistance (D-test). If the organism is resistant to clindamycin it may be necessary to treat the mother with vancomycin. In the U.S., resistance rates are approximately 25–32% for erythromycin and 13–20% for clindamycin (Castor et al., 2008).
Treatment of early onset GBS infection (newborns less than 1 week old): This usually occurs within 24–48 h of birth and most often presents as sepsis or pneumonia. If the infant shows signs of sepsis or respiratory distress they are evaluated and treated with ampicillin and an antibiotic that covers Gram-negative organisms such as Escherichia coli.
Treatment of alpha-hemolytic and nonhemolytic streptococcal infections
Viridans group and S. bovis
Viridans group and S. bovis can cause serious infections such as endocarditis, but are usually susceptible to penicillin. IV penicillin G or ceftriaxone or a combination of β-lactam plus gentamicin for 2–4 weeks is the usual treatment.
Pneumococci
Treatments of choice for SPN are penicillins, macrolides and fluoroquinolones, however antimicrobial resistance in pneumococci, particularly to first-line agents such as penicillin and erythromycin, is increasing in many parts of the world. Resistant strains are more likely to cause invasive pneumococcal disease.
Penicillin resistance. β-Lactams, such as penicillin, act as structural analogues to peptidoglycan precursors, which inhibits cell wall synthesis. Resistance to β-lactams is invariably due to a modification of the target site, namely the PBPs. Those at highest risk for infection with penicillin-resistant pneumococcus are children under 6 years of age, children in day care centers, persons who have previous antibiotic use or are immunocompromised. A recent large surveillance study (n=9692) showed global rates for penicillin-nonsusceptible (intermediate and resistant) SPN are 35–45% (Bouchillon et al., 2010).
Macrolide resistance. Resistance to macrolides such as erythromycin, azithromycin and clarithromycin can be mediated by several different mechanisms. In some strains the target site is modified so that macrolides no longer bind. Such modification is due to the activity of a transposon-mediated enzyme encoded by the ermB gene, which renders the strain resistant not only to macrolides but also to lincosamides and streptogramin B compounds (Robinson et al., 2006). This phenotype is designated MLSB resistance. Another mechanism of macrolide resistance in pneumococci involves the efflux of antibiotics from the bacterial cell and is encoded by the mefA gene. This mechanism generally confers a lower level of resistance to erythromycin than ermB and does not confer resistance to clindamycin and streptogramin B antibiotics.
Fluoroquinolone resistance. SPN strains resistant to fluoroquinolones, such as ciprofloxacin, are also emerging, especially in immunocompromised patients with previous fluoroquinolone use. In fact, previous fluoroquinolone use is the main risk factor for resistance in invasive pneumococcal infections and for colonisation with quinolone nonsusceptible strains of SPN (Jiménez et al., 2005). These strains have mutations in DNA topoisomerase IV (parC and parE) and DNA gyrase genes (gyrA and gyrB). Fluoroquinolone resistance in SPN is not as common as macrolide resistance, however, increasing numbers of pneumococci have reduced susceptibility to ciprofloxacin. Most are still susceptible to newer fluoroquinolones such as levofloxacin, moxifloxacin and gatifloxacin, and may have only one topoisomerase mutation in the parC gene. A recent study (2009–2010) evaluating multiresistant SPN from six European countries showed 99.1% were susceptible to levofloxacin (Pillar et al., 2011).
Recommendations for pneumococcal infections vary depending on patient conditions such as history of antibiotic use, comorbidities and hospitalisation status. Combination therapies are often used and include a respiratory fluoroquinolone or macrolide plus a β-lactam.
Alternative therapy for multiresistant pneumococci (Table 1)
Newer antibiotics against multiresistant SPN include: linezolid (oxazolidinone); ceftobiprole and ceftaroline (broad-spectrum cephalosporins); dalbavancin, telavancin and oritavancin (lipoglycopeptides); tigecycline (glycylcycline); and telithromycin (ketolide).
Table 1 Newer antimicrobial agents with in vitro activity against multidrug-resistant Streptococcus pneumoniae
| Antibiotic | Comments/references |
| Fluoroquinolones | |
| Levofloxacin | Pillar et al. (2011) |
| Moxifloxacin | |
| Oxazolidinones | |
| Linezolid | Jones et al. (2011) |
| Broad-spectrum cephalosporins | |
| Ceftobiprole | Investigational/Farrell et al. (2010) |
| Ceftaroline | Approved in U.S. for CAP in adults/Jacobs et al. (2010) |
| Lipoglycopeptides | |
| Oritavancin | Investigational/Arhin et al. (2009) |
| Dalbavancin | Billeter et al. (2008) |
| Telavancin | Saravolatz et al. (2009; Zhanel et al. (2010 |
| Glycylcyclines | |
| Tigecycline | Carpenter and Chambers (2010) |
| Ketolides | |
| Telithromycin |



