Adult T-cell Leukemia/Lymphoma, HTLV-1+
Carlos E. Bueso-Ramos, MD, PhD
Roberto N. Miranda, MD
Key Facts
Terminology
Peripheral T-cell leukemia/lymphoma caused by human T-cell lymphotropic virus type 1 (HTLV-1)
4 clinical variants are recognized
Acute, lymphomatous, chronic, smoldering
Clinical Issues
Lymphadenopathy that spares the mediastinum
Hepatosplenomegaly, skin lesions
Leukemic involvement, hypercalcemia, lytic lesions
Seropositivity for HTLV-1 can be used as surrogate for HTLV-1 integration into tumor
Useful in areas with low prevalence of HTLV-1 infection
Microscopic Pathology
Diffuse effacement of lymph node architecture
Variable cytologic composition in tissues
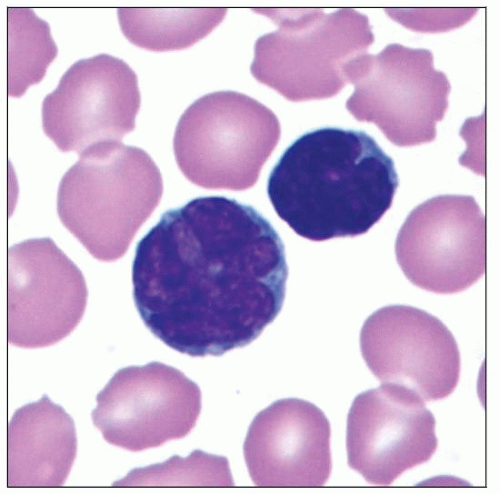
“Flower cells” in peripheral blood
Ancillary Tests
Immunophenotype
CD2(+), CD3(+), CD5(+), TCR-α/β(+)
CD25(+), CCR4(+), FOXP3(+), CD62 (L-selectin)(+)
CD45RO(+); most cases CD4(+), CD8(−)
Complex cytogenetic abnormalities
Monoclonal integration of HTLV-1 into host genome
Monoclonal TCR gene rearrangements
Top Differential Diagnoses
Peripheral T-cell lymphoma, NOS
Angioimmunoblastic T-cell lymphoma
Anaplastic large cell lymphoma
Mycosis fungoides/Sézary syndrome
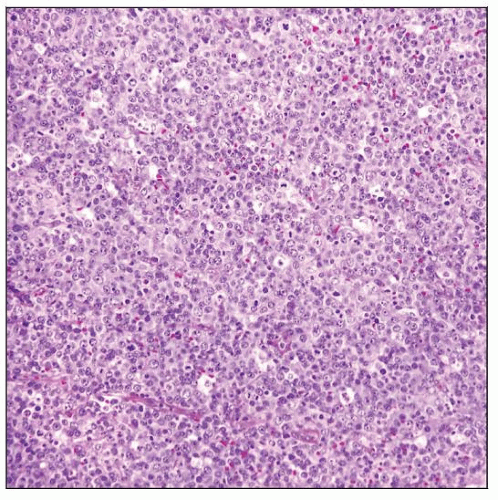 Adult T-cell leukemia/lymphoma (ATLL) diffusely involving lymph node. In this case a “starry sky” pattern can be appreciated, indicating a high cell turnover. |
TERMINOLOGY
Abbreviations
Adult T-cell leukemia/lymphoma (ATLL)
Synonyms
HTLV-1-associated T-cell lymphoma
T-cell lymphoma small cell type or pleomorphic medium and large cell type (HTLV-1[+])
T-cell immunoblastic sarcoma
Definitions
Peripheral T-cell leukemia/lymphoma caused by human T-cell lymphotropic virus type 1 (HTLV-1) infection
4 clinical variants are recognized
Acute, lymphomatous, chronic, smoldering
5th clinical variant has been proposed: Cutaneous
ETIOLOGY/PATHOGENESIS
Infectious Agents
HTLV-1 is a type C retrovirus, delta retrovirus genus
Single strand of RNA that, during infection is
Converted into double strand of DNA in host
Monoclonally integrated into host cell genome
All cells have same site of proviral integration
Pathogenesis
HTLV-1 is spread in 4 general ways
Vertical transmission from mother to child via breast-feeding
Sexual intercourse with infected person
Transfusion of contaminated blood products
Sharing of contaminated needles and syringes among drug users
HTLV-1 can infect immature thymocytes and mature CD4(+) T cells
HTLV-1 infection occurs and spreads via cell-to-cell contact
Genome of HTLV-1 is composed of
Long terminal repeat (LTR) regions at each end
Structural genes: gag, pol, and env
pX region that encodes for tax, rex, p12, p13, p21, and p30 proteins
P40 tax viral protein is needed for HTLV-1 to transform cells in early stages of disease
Many cellular genes are transcriptionally activated by tax
Growth factor interleukin (IL)-2
Its high-affinity receptor α subunit (IL-2Rα; CD25) promotes autocrine stimulation
JAK/STAT pathway is constitutively activated in HTLV-1-infected cells
Tax can repress transcription of genes that
Negatively control cell cycle
Inhibit proteins involved in tumor suppression and DNA repair
Host immunity is involved in control of HTLV-1 infection
Insults to host immune system in viral carrier can result in onset of ATLL
Marked immunodeficiency that results from HTLV-1-infection can lead to opportunistic infections
Molecular Aberrations
HTLV-1 infection alone is insufficient to cause ATLL
Numerous cytogenetic and molecular abnormalities are present
Molecular models suggest 6 or 7 “hits” involved in pathogenesis of full-blown ATLL
CLINICAL ISSUES
Epidemiology
Incidence
HTLV-1 is endemic in
Southwestern Japan, sub-Saharan Africa
Caribbean basin: Jamaica and Martinique
South America: Northern Brazil, Colombia, and French Guyana
Cumulative incidence of ATLL is estimated to be 2.5% among HTLV-1 carriers in Japan
Over 1.2 million persons in Japan are infected with HTLV-1
Prevalence of HTLV-1 infection is very low in North America and Europe
Variable frequency of seroprevalence in various countries probably related to
Genetic predisposition, cultural, and geographical factors
˜ 10% of patients have positive family history
Age
Range: 20-80 years; mean: 58 years
Median age of onset of ATLL is younger in Central and South America, between 40-50 years of age
Gender
M:F ≈ 1.5:1
Site
Lymph nodes
Extranodal sites: Main sites are skin and peripheral blood
Other sites: Spleen, lungs, liver, gastrointestinal (GI) tract, and central nervous system
Presentation
Common widespread lymphadenopathy and peripheral blood involvement
4 clinical variants: Acute, lymphomatous, chronic, and smoldering
Acute variant
˜ 50% of cases in Japan
Leukocytosis, skin rash, and lymphadenopathy
Common peripheral blood involvement and hypercalcemia
Lymphomatous variant
˜ 20% of cases in Japan
Lymphadenopathy and skin lesions
Chronic variant
˜ 20% of cases in Japan
Lymphocytosis and mild organ involvement
Smoldering variant
˜ 5% of cases in Japan
Skin or lung lesions
Up to 5% of atypical lymphocytes in absence of leukocytosis
Skin lesions
Scaly and erythematous rash, cutaneous plaques, or nodules
Acute and lymphomatous variants more frequently associated with skin lesions
T-cell immunodeficiency is common
Associated with Pneumocystis jirovecii pneumonia and strongyloidiasis
Endoscopic Findings
Stomach, colon, and small intestine can be affected
Edema, erosions, or polypoid lesions can be identified
Upper GI tract endoscopy with biopsy is recommended for staging
GI tract involvement is frequent in patients with aggressive ATLL
Laboratory Tests
Seropositivity for HTLV-1 can be used as surrogate for monoclonal integration of virus
Only useful in areas with low prevalence of HTLV-1 infection
Complete blood count: Elevated leukocyte count and circulating neoplastic lymphocytes (leukemic phase)
Elevated serum lactate dehydrogenase level reflects disease burden/activity
Hypercalcemia is more common in patients with acute variant
With or without associated lytic bone lesions
Eosinophilia and neutrophilia are common
Elevated soluble interleukin-2 receptor α-chain levels in patients with aggressive ATLL
Natural History
Patients with chronic or smoldering variant can progress to acute or lymphomatous picture
Treatment
Options, risks, complications
Chronic and smoldering variants
Watchful waiting
Acute and lymphomatous variants
Antiviral agents; chemotherapy
Allogeneic hematopoietic stem-cell transplant
Novel targeted therapies
Drugs
Zidovudine (AZT)/interferon (IFN) α therapy can achieve long-term response
Patients with wild-type p53 and low interferon regulatory factor 4 expression
No standard chemotherapy regimen
Usually transient response or no response
Prognosis
Acute and lymphomatous variants
Median survival time is 13 months
Chronic and smoldering variants have protracted clinical course
IMAGE FINDINGS
Radiographic Findings
Extensive lytic lesions are present in some patients
Skull, pelvis, spine, and long bones can be affected
“Punched-out” lesions similar to multiple myeloma can be found
CT Findings
Computed tomography (CT) scans of body detect sites of nodal and extranodal disease
MICROSCOPIC PATHOLOGY
Cytologic Features
ATLL cells show variable appearances
Irregular/polylobulated nuclei, homogeneous, condensed chromatin, small nucleoli
Agranular basophilic cytoplasm
Lymph Nodes
ATLL initially involves paracortical T-cell zones, leaving B-cell regions unaffected
Subsequently ATLL diffusely replaces lymph node
Lymph nodes involved by ATLL have been subdivided according to cell type and pattern into
Pleomorphic small-cell (usually monotonous)
Pleomorphic medium- and large cell type/pattern; this is most common
Anaplastic large-cell (resembling anaplastic large cell lymphoma)
CD30(+), anaplastic lymphoma kinase(−)
Angioimmunoblastic T-cell lymphoma-like
Medium to large neoplastic cells with abundant clear cytoplasm
Inflammatory cells and proliferation of high endothelial venules
Neoplastic cells are CD3(+), CD10(−), PD-1(−), CXCL13(−)
No proliferation of follicular dendritic cells
Hodgkin lymphoma-like; contains multinucleated HRS-like cells
Epstein-Barr virus positive B cells are interspersed within lesion
Smaller lymphocytes show marked atypia; distinguishing feature from Hodgkin lymphoma
Mitotic and apoptotic rates are variable
Often very high in acute and lymphomatous variants
Inflammatory background including eosinophils is sparse
Peripheral Blood and Bone Marrow
ATLL cells are of intermediate or large size, up to 3x size of normal lymphocytes
Convoluted or multilobulated nuclei, coarse chromatin, and prominent nucleoli
Distinctive appearance of these cells has led to their designation as “flower cells”
Basophilic cytoplasm, with or without vacuoles
In some ATLL patients, neoplastic cells are more uniform in size and shape
Nuclei are smaller and less pleomorphic
Bone marrow involvement may be difficult to identify
Infiltrates of ATLL are usually patchy and interstitial
Diffuse replacement of the medullary space is rare
Increased bone resorption can be seen
Osteoclasts can be increased
Independent poor prognostic factor
Skin
Skin lesions are common in ATLL patients: ˜ 40-70%
Erythematous rash, papules, or tumor nodules
Erythematous lesions tend to be composed of smaller cells in perivascular pattern in dermis
Papules and nodules tend to be composed of larger cells that replace dermis
Epidermotropism, including well-formed Pautrier-like microabscesses, can occur
ANCILLARY TESTS
Immunohistochemistry
Pan-T-cell antigens(+)
CD2, CD3, CD5, and T-cell receptor (TCR)-α/β
CD25(+), CCR4(+), HLA-DR(+), CD62 (L-selectin)(+)
FOXP3(+), which is a marker of regulatory T cells
CD45RO(+); most cases CD4(+), CD8(−)
IRF-4/MUM1(+/−), CD15(−/+), CD30(−/+), CD56(−/+)
Ki-67/MIB-1 shows high proliferation index
TdT(−), ALK1(−), TCL1(−), cytotoxic molecules(−)
B-cell antigens(−), myeloid-associated antigens(−)
Flow Cytometry
ATLL is neoplasm of mature T cells
CD2(+), CD3(+), CD5(+), CD45RO(+), TCR-αβ(+)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree