Adult T-Cell Leukemia/Lymphoma
Definition
Adult T-cell leukemia/lymphoma (ATLL) is a T-cell neoplasm caused by infection by human T-cell lymphotropic virus type 1 (HTLV-1). Four clinical variants are recognized: acute, lymphomatous, chronic, and smoldering (1).
Synonyms
HTLV-1–associated T-cell lymphoma; T-cell lymphoma, small-cell type or pleomorphic medium- and large-cell type (HTLV-1+) (Kiel); T-cell immunoblastic sarcoma (Lukes-Collins); malignant lymphoma, diffuse mixed small- and large-cell, diffuse large-cell, or large-cell immunoblastic (Working Formulation).
Etiology
In 1979, human T-cell lymphotropic virus type 1, identified in a cell line established from a patient with T-cell lymphoma involving skin, was the first oncogenic human virus to be discovered (2). Human T-cell lymphotropic virus type 1 is a type C virus that belongs to the retrovirus family and the delta retrovirus genus. The genome of HTLV-1 is a single strand of RNA that, during infection, is converted into a double strand of DNA (also known as provirus) in the host cell and inserted into the host cell genome.
Approximately 10 to 20 million people in the world are infected by HTLV-1 (3,4). The virus is endemic in southern Japan, sub-Saharan Africa, the Caribbean basin (notably Jamaica and Martinique), and South America (particularly northern Brazil, Colombia, Guyana, and French Guyana) (3). Human T-cell lymphotropic virus type 1 infection is also frequent in native Americans living in the Andes Mountains, in Australian aborigines, and in native populations of Melanesia, Papua New Guinea, Vanuatu, and the Solomon Islands. The prevalence of HTLV-1 infection is very low in North America and Europe, although Romania has been suggested as a potential endemic region. In North America and western Europe, however, large cities with recent immigrants from endemic regions form small pockets with a higher prevalence of HTLV-1 seropositivity (5). It is thought that HTLV-1 first infected humans in Africa approximately 30,000 years ago and spread to the Americas and Japan, in large part via the African slave trade and navigational exploration (6). Genetic predisposition or cultural and geographical factors are also likely to be involved in the different frequencies of seroprevalence in various countries.
Human T-cell lymphotropic virus type 1 virus is spread in four general ways (3). The most common is vertical transmission from mother to child via breast-feeding. In HTLV-1+ mothers, approximately 15% to 25% of children will become infected by virus, depending in part on the mother’s viral load and the duration of breast-feeding. The other means of infection are sexual intercourse with an infected person, transfusion of contaminated cellular blood products, and the sharing of contaminated needles and syringes among drug users.
Human T-cell lymphotropic virus type 1 can cause or is associated with a number of diseases (3,4). It is best known as the cause of ATLL, the focus of this chapter. However, HTLV-1 also is the cause of tropical spastic paraparesis/
HTLV-1–associated myelopathy (TSP/HAM) (7), an inflammatory disease of the white and gray matter of the spinal cord, particularly the lateral and anterior columns, that presents as symmetrical paraparesis of the lower limbs, often with bladder dysfunction. The pathogenesis of TSP/HAM is unknown, but it may be autoimmune in nature. Human T-cell lymphotropic virus type 1 also is reported to be associated with uveitis, infective dermatitis, and autoimmune phenomena (e.g., Sjögren syndrome). The marked immunodeficiency that results from HTLV-1 infection can lead to opportunistic infections, most often by Pneumocystis carinii, Aspergillus fumigatus, Cryptococcus neoformans, Strongyloides stercoralis, Mycobacteria tuberculosis, and cytomegalovirus (3).
HTLV-1–associated myelopathy (TSP/HAM) (7), an inflammatory disease of the white and gray matter of the spinal cord, particularly the lateral and anterior columns, that presents as symmetrical paraparesis of the lower limbs, often with bladder dysfunction. The pathogenesis of TSP/HAM is unknown, but it may be autoimmune in nature. Human T-cell lymphotropic virus type 1 also is reported to be associated with uveitis, infective dermatitis, and autoimmune phenomena (e.g., Sjögren syndrome). The marked immunodeficiency that results from HTLV-1 infection can lead to opportunistic infections, most often by Pneumocystis carinii, Aspergillus fumigatus, Cryptococcus neoformans, Strongyloides stercoralis, Mycobacteria tuberculosis, and cytomegalovirus (3).
Epidemiology
The best epidemiologic data for ATLL are derived from Japan. Over 1.2 million persons in Japan are infected with HTLV-1, and 800 to 1,000 new cases of ATLL occur per year (3,4). All Japanese patients with ATLL are seropositive for anti–HTLV-1 antibodies.
However, Japan is not uniformly affected. The frequency of infection is much higher in southwestern Japan (e.g., Kyushu island), where over 10% of the population shows serologic evidence of infection (3,8). Infection by HTLV-1 is much lower in central and northern Japan (e.g., Honshu and Hokkaido islands). Over 50% of cases of ATLL in Japan occur in Kyushu (9). The mean age of ATLL is 60 years in Japan, consistent with a long latency period from time of infection by HTLV-1 to development of ATLL (8,9,10). In Kyushu, the age-specific incidence rate increases with age until 70 years, when it then drops sharply (9). The cumulative incidence of ATLL in viral carriers in Japan is estimated to be 3% to 5% for men and 1% to 2% for women (8). This translates into annual incidence rates of approximately 40 and 26 per million for men and women, respectively (9). The male-to-female ratio is approximately 1.5:1 (1,10). There is also evidence of familial clustering of cases of ATLL in Japan, with approximately 10% of patients having a positive family history and occasional families being devastated by this disease (9,11).
The epidemiologic features of ATLL in other parts of the world are less well known. The median age of onset of ATLL is younger in Central and South America, between 40 and 50 years of age. Adult T-cell leukemia/lymphoma can rarely occur in children, and this seems to be more common in Brazil (12). The male-to-female ratio in the Caribbean and Central and South America is relatively lower, at 1.2:1 (3).
Pathogenesis
Human T-cell lymphotropic virus type 1 retrovirus and its genome is a single strand of RNA, but this is converted to a provirus (double strand of DNA) in the host and randomly inserted into the host cell genome (3,13). Thus, ATLL cells contain proviral HTLV-1 integrated into their DNA. The virus is not simply a passenger but is present in the cell before neoplastic transformation. In addition, in a case of ATLL, all cells have the same site of proviral integration, further indicating that the ATLL arose from a single virally infected progenitor cell.
Human T-cell lymphotropic virus type 1 infection occurs and spreads via cell-to-cell contact, unlike other viruses that can infect as free virus (e.g., HIV). Human T-cell lymphotropic virus type 1 can infect immature thymocytes as well as any mature T cells, but it has a preference for infecting CD4+ T cells. Human T-cell lymphotropic virus type 1 does not multiply within an infected cell. Thus, the total load of HTLV-1 provirus in a patient depends, in large part, on the proliferation of infected cells.
The genome of HTLV-1 is composed of two long terminal repeat (LTR) regions at each end; the structural genes gag, pol, and env; and the pX region (13). The latter is important in pathogenesis as it encodes for the tax, rex, p12, p13, p21, and p30 proteins. Human T-cell lymphotropic virus type 1 does not contain a transforming oncogene; instead, the regulatory protein TAX is needed for HTLV-1 to transform cells in the early stages, but it is not transcribed in a substantial subset of ATLLs, implying that TAX is not needed once transformation has taken place. Its absence also allows the neoplastic cells to escape immune surveillance by cytotoxic T cells. By binding with host cell proteins, TAX can repress the transcription of genes that negatively control the cell cycle (e.g., cyclin-dependent kinase inhibitors), inhibit proteins involved in tumor suppression and DNA repair (e.g., p53), bypass cell cycle checkpoints (e.g., by activating cyclin-dependent kinases), and inhibit the tendency of virally infected cells to undergo apoptosis. Regarding the latter, TAX has been shown to activate both the nuclear factor (NF)-κB and the phosphatidylinositol 3 kinase (PI3K)/AKT cellular pathways. Many cellular genes are transcriptionally activated by TAX, including the growth factor interleukin (IL)-2 and its high-affinity receptor α subunit (IL-2Rα; CD25). CD25 promotes autocrine stimulation. The JAK/STAT pathway has been shown to be constitutively activated in HTLV-1–infected cells (14). Human T-cell lymphotropic virus type 1 infection also causes chromosomal instability leading to tumor cell aneuploidy.
It is important to not overemphasize the role of TAX. Other genes are very important in the pathogenesis of ATLL, as reviewed by Matsuoka and Jeang (13). The 3′ LTR region, the hbz gene encoded by the minus RNA strand of the provirus, possibly microRNAs, and other genes play important roles in the pathogenesis of ATLL. Human T-cell lymphotropic virus type 1 infection alone is insufficient to cause ATLL. Other genetic events are required for ATLL to arise, and this fact explains the long latency interval from initial infection to development of neoplasm. Host immunity is also involved in the control of HTLV-1 infection, and insults to the host immune system in a viral carrier can result in the onset of ATLL (3,13,15).
Clinical Features
Four well-established, clinical variants of ATLL occur: acute, lymphomatous, chronic, and smoldering (1,3,13). A fifth variant also has been proposed: cutaneous ATLL (16). In Japan, the acute variant represents approximately 55% of all ATLL patients. The remaining patients have either the lymphomatous (20%), chronic (20%), or smoldering (5%) variants (16). Prognosis is much worse for patients with the acute or lymphomatous variants compared with patients who have the chronic and smoldering variants. However, patients with chronic and smoldering variants can evolve into the acute or lymphomatous variants of ATLL (1,3).
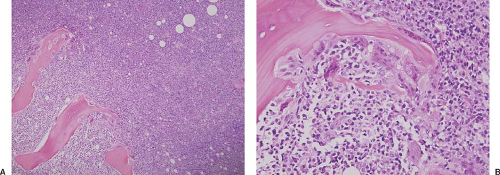
In the acute variant of ATLL, affected patients have an overt leukemic phase of disease, often with an elevated leukocyte count and circulating neoplastic lymphocytes, associated constitutional symptoms, elevated serum lactate dehydrogenase level, generalized lymphadenopathy, and hepatosplenomegaly (clinical stage IV). The leukocyte count can rise to extraordinarily high levels (e.g., 1–2 × 105), with most of these cells being neoplastic lymphocytes. Hypercalcemia is common and often associated with lytic bone lesions; it is thought to be the result of increased osteoclastic activity, perhaps related to a parathyroid-hormone–like substance that is frequently increased in these patients (Fig. 71.1) (3). Eosinophilia and neutrophilia are often present, but without substantial anemia or
thrombocytopenia (16). Acute ATLL is frequently associated with a scaly and erythematous rash, cutaneous plaques, or nodules. Other sites of disease include the lungs, liver, gastrointestinal tract, and central nervous system (usually in the form of lymphomatous meningitis).
thrombocytopenia (16). Acute ATLL is frequently associated with a scaly and erythematous rash, cutaneous plaques, or nodules. Other sites of disease include the lungs, liver, gastrointestinal tract, and central nervous system (usually in the form of lymphomatous meningitis).
The clinical findings in patients with the lymphomatous variant of ATLL greatly overlap with the acute form, as patients have systemic disease. However, patients with the lymphomatous variant do not have a leukemic phase, and hypercalcemia is relatively less common. In the chronic variant of ATLL, skin lesions are common, most often as an exfoliative rash. The leukocyte count is usually mildly elevated, and neoplastic lymphocytes are relatively easily identified on the blood smear (>5% or 10% of the differential count) (1). Mild lymphadenopathy and hepatosplenomegaly are often present, and hypercalcemia can occur. The smoldering variant of ATLL is characterized by a normal leukocyte count with occasional neoplastic cells in the blood (<3% or 5% of the differential count). Skin lesions are very common, but hypercalcemia, lymphadenopathy, and hepatosplenomegaly are absent. In the recently proposed cutaneous variant of ATLL, only skin lesions occur without evidence of leukemic phase, lymphadenopathy, hepatosplenomegaly, or hypercalcemia (16,17).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree