INTRODUCTION
Adenoviruses can replicate and produce disease in the respiratory, gastrointestinal, and urinary tracts and in the eye. Many adenovirus infections are subclinical, and virus may persist in the host for months. About one-third of the 57 known human serotypes are responsible for most cases of human adenovirus disease. A few types serve as models for cancer induction in animals. Adenoviruses are especially valuable systems for molecular and biochemical studies of eukaryotic cell processes. They are also useful vectors for gene therapy approaches.
PROPERTIES OF ADENOVIRUSES
Important properties of adenoviruses are listed in Table 32-1.
| Virion: Icosahedral, 70–90 nm in diameter, 252 capsomeres; fiber projects from each vertex |
| Composition: DNA (13%), protein (87%) |
| Genome: Double-stranded DNA, linear, 26–45 kbp, protein bound to termini, infectious |
| Proteins: Important antigens (hexon, penton base, fiber) are associated with the major outer capsid proteins |
| Envelope: None |
| Replication: Nucleus |
| Outstanding characteristic: Excellent models for molecular studies of eukaryotic cell processes |
Adenoviruses are 70–90 nm in diameter and display icosahedral symmetry, with capsids composed of 252 capsomeres. There is no envelope. Adenoviruses are unique among icosahedral viruses in that they have a structure called a “fiber” projecting from each of the 12 vertices, or penton bases (Figures 32-1 and 32-2). The rest of the capsid is composed of 240 hexon capsomeres. The hexons, pentons, and fibers constitute the major adenovirus antigens important in viral classification.
FIGURE 32-1
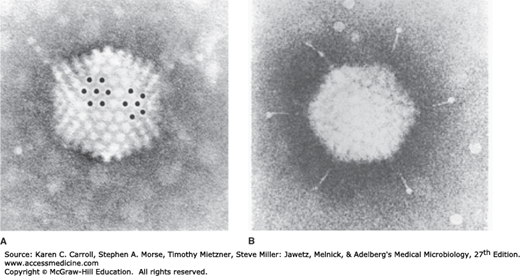
Electron micrographs of adenovirus. A: The viral particle displays cubic symmetry and is nonenveloped. A hexon capsomere (surrounded by six identical hexons) and a penton capsomere (surrounded by five hexons) are marked with dots. B: Note the fiber structures projecting from the vertex penton capsomeres (285,000×). (Reproduced with permission from Valentine RC, Pereira HG: Antigens and structure of the adenovirus. J Mol Biol 1965;13:13.)
FIGURE 32-2
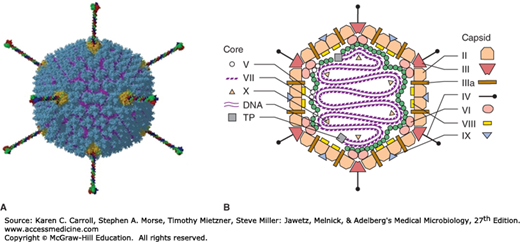
Models of the adenovirus virion. A: A three-dimensional image reconstruction of an intact adenovirus particle showing fibers projecting from the penton bases. (Reproduced with permission from Liu H, Wu L, Zhou ZH: Model of the trimeric fiber and its interactions with the pentameric penton base of human adenovirus by cryo-electron microscopy. J Mol Biol 2011;406:764. [Graphical abstract.] Copyright Elsevier.) B: A stylized section of the adenovirus particle showing polypeptide components and DNA. No real section of the icosahedral virion would contain all components. Virion constituents are designated by their polypeptide numbers with the exception of the terminal protein (TP). (Reproduced with permission from Stewart PL, Burnett RM: Adenovirus structure as revealed by x-ray crystallography, electron microscopy and difference imaging. Jpn J Appl Phys 1993;32:1342.)
The DNA genome (26–45 kbp) is linear and double stranded. The GC content of the DNA is lowest (48–49%) in group A (types 12, 18, and 31) adenoviruses, the most strongly oncogenic types, and ranges as high as 61% in other types. This is one criterion used in grouping human isolates. A virus-encoded protein is covalently linked to each 5′ end of the linear genome. The DNA can be isolated in an infectious form, and the relative infectivity of that DNA is reduced at least 100-fold if the terminal protein is removed by proteolysis. The DNA is condensed in the core of the virion; a virus-encoded protein, polypeptide VII (see Figure 32-2B), is important in forming the core structure.
There are an estimated 11 virion proteins; their structural positions in the virion are shown in Figure 32-2B. Hexon and penton capsomeres are the major components on the surface of the virus particle. There are group- and type-specific epitopes on both the hexon and fiber polypeptides. All human adenoviruses display this common hexon antigenicity. Pentons occur at the 12 vertices of the capsid and have fibers protruding from them. The penton base carries a toxin-like activity that causes rapid appearance of cytopathic effects and detachment of cells from the surface on which they are growing. Another group-reactive antigen is exhibited by the penton base. The fibers contain type-specific antigens that are important in serotyping. Fibers are associated with hemagglutinating activity. Because the hemagglutinin is type specific, hemagglutination-inhibition tests are commonly used for typing isolates. It is possible, however, to recover isolates that are recombinants and give discordant reactions in antibody neutralization and hemagglutination-inhibition assays.
Adenoviruses have been recovered from a wide variety of species and grouped into five genera. All of the human adenoviruses are classified in the Mastadenovirus genus. At least 57 distinct antigenic types have been isolated from humans and many other types from various animals.
Human adenoviruses are divided into seven groups (A–G) on the basis of their genetic, physical, chemical, and biologic properties (Table 32-2). Adenoviruses of a given group have fibers of a characteristic length, display considerable DNA homology (>85%, compared with <20% of members of other groups), and exhibit similar capacities to agglutinate erythrocytes from either monkeys or rats. Members of a given adenovirus group resemble one another in the GC content of their DNA and in their potential to produce tumors in newborn rodents. Importantly, viruses within a group tend to behave similarly with respect to epidemiologic spread and disease association.
| Group | Serotypes | Hemagglutination | Percentage of G + Ca in DNA | Oncogenic Potential | ||
|---|---|---|---|---|---|---|
| Group | Result | Tumorigenicity in Vivob | Transformation of Cells | |||
| A | 12, 18, 31 | IV | None | 48–49 | High | + |
| B | 3, 7, 11, 14, 16, 21, 34, 35, 50, 55 | I | Monkey (complete) | 50–52 | Moderate | + |
| C | 1, 2, 5, 6, 57 | III | Rat (partial) | 57–59 | Low or none | + |
| D | 8–10, 13, 15, 17, 19, 20, 22–30, 32, 33, 36–39, 42–49, 51, 53, 54, 56 | II | Rat (complete) | 57–61 | Low or nonec | + |
| E | 4 | III | Rat (partial) | 57 | Low or none | + |
| F | 40, 41 | III | Rat (partial) | 57–59 | Low or none | + |
| G | 52 | unknown | 55 | unknown | unknown | |
Adenoviruses replicate well only in cells of epithelial origin. The replicative cycle is sharply divided into early and late events. The carefully regulated expression of sequential events in the adenovirus cycle is summarized in Figure 32-3. The distinction between early and late events is not absolute in infected cells; early genes continue to be expressed throughout the cycle; a few genes begin to be expressed at “intermediate” times; and low levels of late gene transcription may occur soon after infection.
FIGURE 32-3
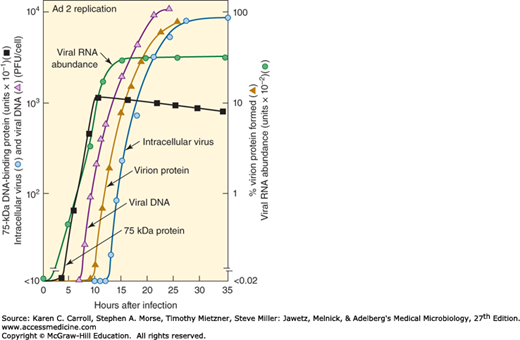
Time course of adenovirus replication cycle. The time between infection and the first appearance of progeny virus is the eclipse period. Note the sequential regulation of specific events in the virus replication cycle. PFU means plaque-forming unit, a measure of infectious virus. (Courtesy of M Green.)
The virus attaches to cells via the fiber structures. The host cell receptor for some serotypes is CAR (coxsackie–adenovirus receptor), a member of the immunoglobulin gene superfamily. The interaction of the penton base with cellular integrins after attachment promotes the internalization step. Adsorption and internalization are separate steps in the adenovirus infection process, requiring the interaction of fiber and penton proteins with different cellular target proteins. Adsorbed virus is internalized into endosomes; the majority of particles (~90%) move rapidly from endosomes into the cytosol (half-life ~5 minutes) by a process triggered by the acidic pH of the endosome. Microtubules are probably involved in the transport of virus particles across the cytoplasm to the nucleus. Uncoating commences in the cytoplasm and is completed in the nucleus, with release of viral DNA perhaps occurring at the nuclear membrane. Uncoating is an organized, sequential process that systematically breaks down the stabilizing interactions that were established during maturation of the virus particle.
The steps that occur before the onset of viral DNA synthesis are defined as early events. The goals of the early events are to induce the host cell to enter the S phase of the cell cycle to create conditions conducive to viral replication, to express viral functions that protect the infected cell from host defense mechanisms, and to synthesize viral gene products needed for viral DNA replication.
The early (“E”) transcripts come from seven widely separated regions of the viral genome and from both viral DNA strands. More than 20 early proteins, many of which are nonstructural and are involved in viral DNA replication, are synthesized in adenovirus-infected cells. The E1A early gene is especially important; it must be expressed for the other early regions to be transcribed. Modulation of the cell cycle is accomplished by the E1A gene products. The E1B early region encodes proteins that block cell death (apoptosis) occurring as a result of E1A functions; this is necessary to prevent premature cell death that would adversely affect virus yields. The E1A and E1B regions contain the only adenovirus genes necessary for cell transformation; those gene products bind cellular proteins (eg, pRb, p300, p53) that regulate cell cycle progression. The early proteins are represented by the 75-kDa DNA-binding protein shown in Figure 32-3.
Viral DNA replication takes place in the nucleus. The virus-encoded, covalently linked terminal protein functions as a primer for initiation of viral DNA synthesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree