Adenosis and Microglandular Adenosis
ADENOSIS
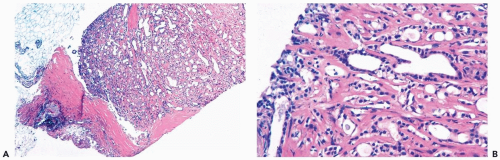
Adenosis is a proliferative lesion largely derived from the terminal duct-lobular unit. Epithelial and myoepithelial cells participate in adenosis, which is characteristically a lobulocentric lesion (Fig. 6.1). Adenosis usually occurs as part of a spectrum of proliferative abnormalities commonly referred to as fibrocystic changes. The entire complex may produce a palpable mass that is usually not attributable to only the adenosis component. When limited to isolated lobules that are not part of fibrocystic change, adenosis is a microscopic lesion that comes to attention clinically, if it contains calcifications that are detected by mammography.
A palpable mass formed largely or completely by adenosis occurs when there is fusion of the affected lobules to form an adenosis tumor (Fig. 6.2). Patients with an adenosis tumor are almost always premenopausal, averaging about age 30 at diagnosis (1,2). The tumor is usually smaller than 2 cm and easily mistaken for a fibroadenoma (1,3). Nonpalpable adenosis tumors that contain calcifications may be detected by mammography. Sonography reveals a solid, well-defined tumor (2).
Adenosis tends to have a more prominent glandular pattern in premenopausal women, whereas sclerosis and loss of gland formation are conspicuous after the menopause. In some patients, there is very little variability in the spectrum of adenosis, but others exhibit diverse patterns.
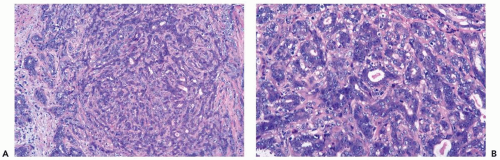
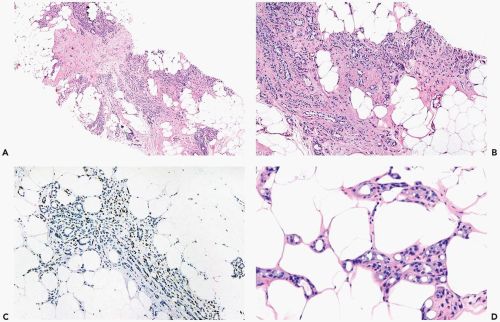
The most cellular type of adenosis, referred to as florid adenosis, is the growth pattern usually present in adenosis tumors. Proliferation of ductules and lobular glands severely distorts and usually effaces the architecture of the underlying lobules. The hyperplastic structures elongate, becoming tortuous and entwined in a fashion that results in many more ductular cross sections than are present in an anatomically normal lobule (Fig. 6.3). In the plane of section, the complex proliferative structure has a swirling pattern, punctuated by glands cut transversely that have round, open lumina. The majority of the ductular structures cut tangentially or longitudinally have elongated lumina, usually with rounded but sometimes with angular contours.
Epithelial cells lining the tubules and glands are flattened, cuboidal, or slightly columnar, and arranged in one or two layers surrounded by myoepithelial cells. Increase in cell size and nuclear pleomorphism can be found in florid adenosis, especially during pregnancy or lactation. Intracytoplasmic mucin vacuoles and signet ring cells are not present in the benign glandular epithelium of florid adenosis. Luminal secretion may undergo calcification, but this is less common and less extensive than in sclerosing adenosis. Hyperplastic change in the epithelial component of florid adenosis is mirrored in hyperplasia of the myoepithelium. Mitoses in epithelial and myoepithelial cells are very infrequent, but they may be more evident during pregnancy (Fig. 6.4). Apocrine metaplasia occurs in florid adenosis. Florid adenosis may surround nerves in adjacent breast parenchyma.
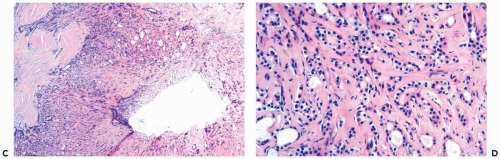
In sclerosing adenosis there is preferential preservation of myoepithelial cells with variable atrophy of epithelial cells accompanied by lobular fibrosis (Fig. 6.5). The swirling lobulocentric pattern encountered in adenosis is retained, but epithelial cells are less conspicuous, and the tubular structures tend to be more attenuated. Some examples of sclerosing adenosis are not limited to a lobulocentric pattern. When this occurs, the proliferating benign glands have an infiltrative pattern in the stroma (Fig. 6.6)
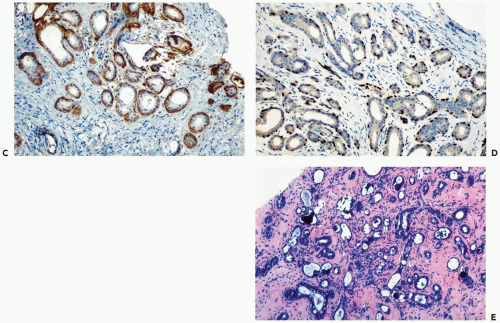
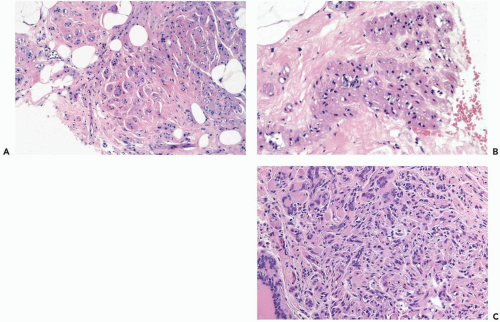
and fat (Fig. 6.7) that can be mistaken for invasive carcinoma (Fig. 6.8), especially in fragmented needle core biopsy samples that lack the orientation provided by surrounding tissue in sections from a surgical biopsy. Epithelial cells may be markedly reduced in number, or even absent, leaving compressed elongated myoepithelial cells surrounded by basement membranes. The persisting myoepithelial cells with a pronounced spindle shape or myoid phenotype (Fig. 6.9) are strongly immunoreactive with cytoplasmic markers of smooth muscle differentiation and for the nuclear marker p63. Calcifications become more numerous with increasing sclerosis and thickening of basement membranes (Fig. 6.10).
and fat (Fig. 6.7) that can be mistaken for invasive carcinoma (Fig. 6.8), especially in fragmented needle core biopsy samples that lack the orientation provided by surrounding tissue in sections from a surgical biopsy. Epithelial cells may be markedly reduced in number, or even absent, leaving compressed elongated myoepithelial cells surrounded by basement membranes. The persisting myoepithelial cells with a pronounced spindle shape or myoid phenotype (Fig. 6.9) are strongly immunoreactive with cytoplasmic markers of smooth muscle differentiation and for the nuclear marker p63. Calcifications become more numerous with increasing sclerosis and thickening of basement membranes (Fig. 6.10).
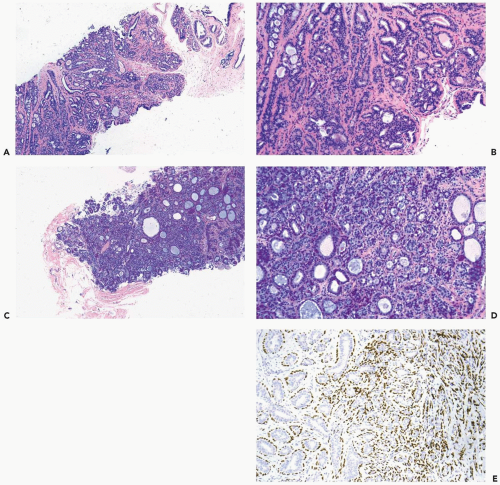
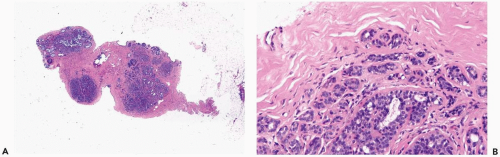 Figure 6.1 Adenosis. A, B. Multiple nodules of adenosis are shown in this needle core biopsy specimen. Larger nodules are the result coalescent lobules, such as the one shown (B). |
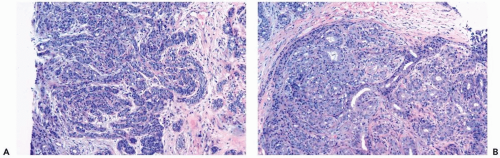 Figure 6.3 Florid adenosis. A, B. Elongated, hyperplastic entwined adenosis glands and myoepithelium are shown in a needle core biopsy specimen. |
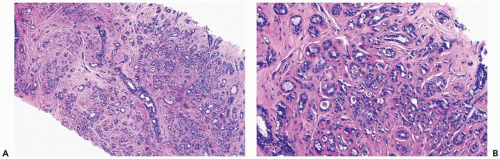 Figure 6.5 Sclerosing adenosis. A, B. A needle core biopsy specimen from a confluent lesion showing stromal fibrosis, thickened periglandular basement membranes, and atrophy of some glands. |
Apocrine metaplasia is relatively common in adenosis, a configuration that has been referred to as apocrine adenosis (3,4) (Fig. 6.11). The cytologic appearance of apocrine adenosis is quite varied (5). In some cases, the cells have conventional pink, finely granular apocrine cytoplasm, and round, regular nuclei. Atypical features include clearing or vacuolization of the cytoplasm as well as nuclear pleomorphism
and hyperchromasia (Fig. 6.12). Mitotic figures are very uncommon in atypical apocrine adenosis.
and hyperchromasia (Fig. 6.12). Mitotic figures are very uncommon in atypical apocrine adenosis.
Oncoprotein expression has been found in apocrine adenosis, including c-erbB2 (55.6%), p53 (27.8%), Bax (33.3%) and c-myc (100%) (6). None of the 18 samples studied was reactive for Bcl-2. Reactivity for these markers was seen in apocrine adenosis with and without atypia. It has not been determined that oncoprotein expression in apocrine adenosis is associated with a predisposition to develop carcinoma independent from the presence of histologic atypia.
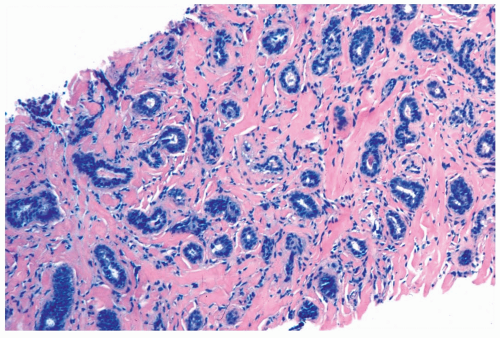
Perineural invasion has been found in 1% to 2% of breast specimens composed of benign proliferative changes, including sclerosing adenosis (Fig. 6.13) (7




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree