Adenocarcinoma and Related Tumors of the Uterine Cervix
EPIDEMIOLOGY
Adenocarcinomas derived from the endocervical epithelium constitute approximately 10% to 15% of all invasive cancers of the cervix. There is suggestive evidence that the frequency of endocervical adenocarcinoma may be on the rise (Kjaer and Brinton, 1993). It is possible that a better recognition of this group of lesions combined with a reduction in the rate of invasive squamous cancer may account for some of the increase. The increased frequency may be limited to geographic regions: a marked increase has been observed in Australia, United Kingdom and Norway, whereas none has been observed in Italy (Debate, 1999). In the United States, there is some evidence of increase in young women (Peters et al, 1986; Schwartz and Weiss, 1986; Horowitz et al, 1988). The disease occurs in adult women of all ages but is most common in women in their late 40s or early 50s.
Dallenbach-Hellweg (1981) proposed a possible relationship between endocervical adenocarcinoma and long-term use of oral contraceptives containing progesterone. Peters et al (1986), Jones and Silverberg (1989), Brinton et al (1990), and Ursin et al (1994) found some evidence in support of this concept. Because of a possible association of all forms of carcinoma of the cervix with the use of oral contraceptives (see Chap. 10), the selective increase of adenocarcinomas in this group of patients has not been documented in a persuasive fashion. Horowitz et al (1988) and Parazzini et al (1988) failed to observe significant epidemiologic differences between women with squamous carcinoma of the cervix and those with adenocarcinoma of the cervix. In fact, the coexistence of precancerous squamous lesions or invasive squamous cancer may be observed in nearly one half of patients with endocervical adenocarcinoma, suggesting a common denominator for these lesions. This common denominator may be human papillomaviruses, particularly types 16 and 18, which are frequently observed in endocervical neoplastic lesions (Wilczynski et al, 1988; Tase et al, 1989; Fransworth et al, 1989; Nielson et al, 1990; Duggan et al, 1995; Iwasawa et al, 1996; Riethdorf et al, 2002) (for discussion of the human papillomavirus, see Chap. 11). Endocervical adenocarcinoma may also be synchronous with endometrial cancer (Friedell and McKay, 1953) and with ovarian carcinoma (Livolsi et al, 1983).
SEQUENCE OF NEOPLASTIC EVENTS
It is generally assumed that the sequence of events in the development of endocervical adenocarcinoma of the uterine cervix is similar to squamous carcinoma, described in the preceding chapter. Theoretically at least, minor morphologic abnormalities of endocervical epithelium, variously termed as “atypia” or “dysplasia,” should precede the development of the true precursor lesions—the adenocarcinomas in situ—which, in turn, should lead to microinvasive and fully invasive cancers. The term endocervical carcinoma in situ, first introduced by Friedell and McKay (1953), has been enshrined in the 2001 Bethesda System of reporting (see Chap. 11). Documenting the sequence of neoplastic events in the endocervix poses problems because, except for its lowest segment, the endocervical canal cannot be visualized by colposcopy and, therefore, cytologic sampling cannot be targeted. Also, in spite of numerous efforts, the morphologic recognition of sequential abnormalities of the endocervical cells is much more difficult than in squamous cells (Lee et al, 2000). The issue is complicated still further by the fact that at least half of endocervical adenocarcinoma is associated with squamous or epidermoid neoplasia in various forms that may have common cytologic features with endocervical neoplasia. To simplify the discussion of a difficult topic, this chapter will begin with a discussion of histology and cytology of invasive endocervical adenocarcinoma, followed by a discussion of precursors.
INVASIVE ENDOCERVICAL ADENOCARCINOMA
Several elaborate systems of classification of endocervical adenocarcinoma have been proposed and recently summarized (Zaino, 2000; Young and Clement, 2002). Because the prognosis of these tumors depends more on stage of disease than histologic presentation (Berek et al, 1984; Kilgore et al, 1988), the simplest classification is their subdivision into common and less common or rare types of tumors.
Among the common types are:
Adenocarcinoma mimicking normal endocervical glands
Mucus-producing adenocarcinomas
Endometrioid carcinomas
Poorly differentiated carcinomas
Synchronous adenocarcinoma and squamous carcinoma
Less common or rare are:
Adenosquamous carcinoma
Villoglandular papillary adenocarcinomas
Adenoma malignum (minimal deviation adenocarcinoma)
Glassy-cell carcinoma
Mucoepidermoid carcinomas
Clear-cell carcinoma (see Chap. 14)
Extremely rare adenocarcinomas
Common Types of Well-Differentiated Adenocarcinoma
Histology
Tumors Mimicking Endocervical Glands
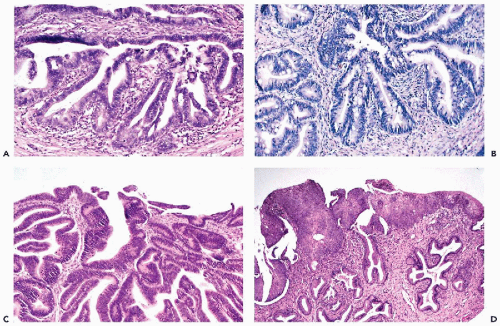
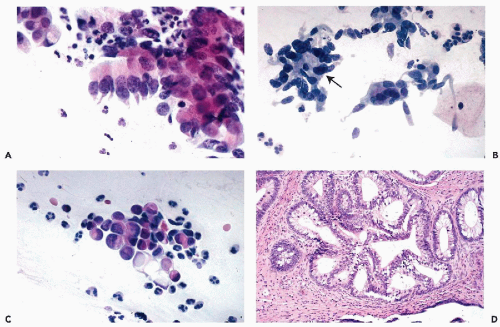
The tumors infiltrate the stroma of the cervix and form glands similar to normal endocervical glands, although the glands vary in size and have irregular configuration. Papillary projections, on the surface of the tumor and within the malignant glands, are fairly common. The malignant glands are lined by one or more layers of tumor cells that are either columnar or cuboidal in shape, have an opaque, granular cytoplasm, and show significantly enlarged, hyperchromatic, coarsely granular nuclei, sometimes provided with large nucleoli (Fig. 12-1A). Histologic diagnosis of the well-differentiated endocervical adenocarcinomas may occasionally cause diagnostic problems because the glands may be mistaken for normal endocervical tissue, particularly in scanty biopsies or endocervical curettage material.
A number of benign variants or benign abnormalities of the endocervical glands may be mistaken for adenocarcinoma. Microglandular hyperplasia, occurring mainly in women with progesterone exposure, endocervical tunnel clusters, mesonephric hyperplasia, and endocervicosis were discussed in Chapter 10. They all have, in common, formation of glandular structures lined by bland benign cuboidal or columnar cells and, regardless of other opinions, are not recognizable in cytologic preparations, contrary to endocervical adenocarcinoma. Thus, cytologic samples may be of significant help in the interpretation of small biopsies of cervix.
A very rare lesion termed microcystic endocervical adenocarcinoma mimics benign lesions of the endocervix but the cysts are lined by clearly malignant cells. In some of these patients, malignant cells may be observed in cervical smears but the precise type of lesion cannot be established (Tambouret et al, 2000).
Although many observers consider all adenocarcinomas derived from endocervical glands as mucinous or mucus-producing, it has been my experience that there is little evidence of mucus production in the most common adenocarcinomas wherein it is usually confined to a few scattered cells. Therefore, endocervical adenocarcinomas with a substantial component of mucus-producing cells, are separated out. This subdivision is of value in the interpretation of cytologic findings.
Mucus-Producing Adenocarcinomas
In these tumors, a substantial proportion of cells lining the glands resemble goblet- or intestinal cells, characterized by markedly distended clear cytoplasm filled with mucus (Fig. 12-1B). Some of these tumors may mimic intestinal cancers because of the presence of occasional Paneth cells with cytoplasmic granules. Signet-ring types of cancer cells may sometimes occur.
Endometrioid Type of Carcinomas
These tumors form glands lined by one or more cuboidal cancer cells, mimicking a similar tumor of endometrial origin (Fig. 12-1C). Mucus production in the tumor cells is either absent or very limited. The nuclear features of the
tumors are similar to those of the adenocarcinoma of endocervical type, described above. For a detailed morphologic description of endometrioid carcinomas, see Chap. 13. Some of the endocervical lesions may be traced to foci of endocervical endometriosis. Others probably represent a histologic variant of endocervical adenocarcinoma. In some cases, curettage may be required to determine the origin of the tumor in the endocervix or the endometrium.
tumors are similar to those of the adenocarcinoma of endocervical type, described above. For a detailed morphologic description of endometrioid carcinomas, see Chap. 13. Some of the endocervical lesions may be traced to foci of endocervical endometriosis. Others probably represent a histologic variant of endocervical adenocarcinoma. In some cases, curettage may be required to determine the origin of the tumor in the endocervix or the endometrium.
As mentioned, these three dominant tumor types are associated with either precursor lesions (CIN) or invasive squamous carcinomas which occur in about 50% of cases (Fig. 12-1D). The impact of this association on cytology will be discussed later in this chapter.
Cytology
Cytologic presentation of endocervical adenocarcinoma has been influenced by the widespread use of endocervical brushes. A large number of papers, many cited in this text, described and analyzed the abnormalities of endocervical cells in minute details that allegedly led to a more precise assessment of the sequence of neoplastic events in the endocervix. Following the example of Rosenthal et al (1982), Van Aspert-van Erp et al (1995, 1997), in a series of elaborate studies, analyzed at great length numerous visual and computer-generated features of endocervical cells in “endocervical columnar cell intraepithelial neoplasia” (ECCIN), ranging from mild to moderate to severe atypia, endocervical adenocarcinoma in situ, and invasive adenocarcinoma. When the numerous criteria established in these studies were tested for reproducibility, only a few of them proved to be of practical diagnostic value. They were essentially the same abnormalities of endocervical cells that have been previously recognized as consistent with adenocarcinoma. Unfortunately, the analysis of abnormalities of the endocervical cells is further complicated by benign atypias occurring in these cells, discussed in Chapter 10 and later in this chapter. If the endocervical brush-induced sampling artifacts are added to the mix, it becomes evident that the topic of abnormalities of endocervical cells may be extremely complex and the elaborate studies have been of limited value in the practice of cytopathology. ThinPrep liquid preparations of cervical samples have a significant effect on morphology of endocervical cells. The nuclei are generally smaller (shrunken) and the nucleoli are often evident in benign cells (Johnson and Rahemtulla, 1999; Selvaggi, 2002).
Cells of Well-Differentiated Invasive Endocervical Adenocarcinoma
It is virtually impossible to separate the three main types of endocervical adenocarcinoma from each other in cytologic preparations, although, occasionally, mucus-producing adenocarcinomas
may shed cells suggestive of this tumor type (see below). In the cervical material, the smear background often shows blood, necrosis, and cell debris.
may shed cells suggestive of this tumor type (see below). In the cervical material, the smear background often shows blood, necrosis, and cell debris.
In samples obtained by either cervical scrapers or endocervical brushes, the following features may be observed:
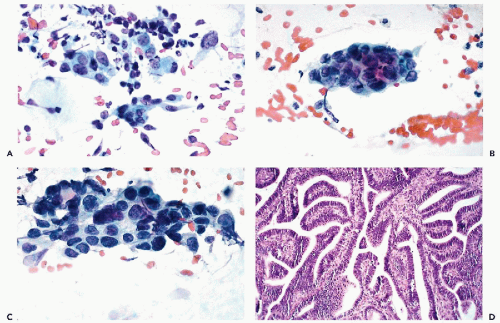
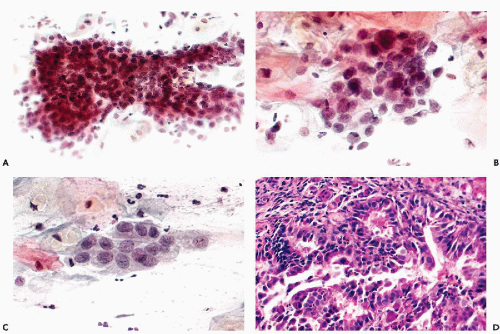
The dominant cancer cells are usually columnar, although often larger or smaller than normal endocervical cells. They have opaque and granular or clear cytoplasm and abnormal nuclei. The nuclear changes comprise enlargement, hyperchromasia, coarse granulation of chromatin and sometimes large, irregular, and multiple nucleoli (Figs. 12-2A and 12-3A).
The cancer cells often form spherical or oval clusters of superimposed cancer cells, corresponding to tumor papillae (Figs. 12-2B,C and 12-3B). At the periphery of such clusters, the columnar configuration of the component cells may be observed. On careful focusing, gland formation within the clusters can be observed.
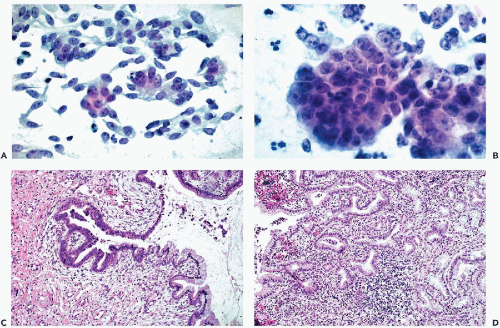
In yet other cases, mitotic figures and apoptotic break-up of nuclei may occur (Fig. 12-4A). Occasionally, large malignant cells, without distinguishing features, may be noted (Fig. 12-4B,C).
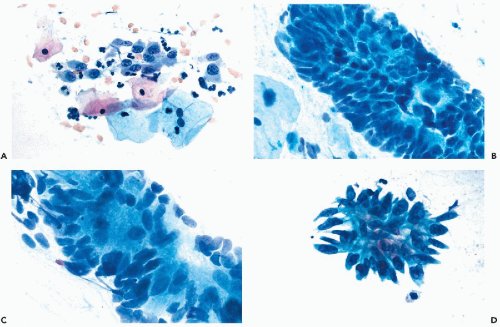
The cancer cells are often arranged in parallel clusters (palisading), reflecting the arrangement of the tumor cells on the surface epithelium (Fig. 12-5A).
They may be arranged around a central lumen (rosettes), reflecting the tendency of the tumor cells to form glands (Fig. 12-5B).
Approximately spherical “signet ring” cancer cells are characteristic of mucus-producing adenocarcinoma. Such cells have a large, peripheral nucleus and a cytoplasm with a large, mucus-containing single vacuole or several smaller vacuoles (Fig. 12-5C).
It is not uncommon to observe, in cervical smears of endocervical adenocarcinomas, a few dysplastic (dyskaryotic) or cancer cells of squamous type. These reflect abortive forms ofsquamous carcinoma, which maybe associated with endocervical adenocarcinoma (see below). Very rarely, similar findings may be observed in adenoacanthomas of the endometrium (see Chap. 13).
In samples obtained by endocervical brushes, additional features of such tumors can be noted:
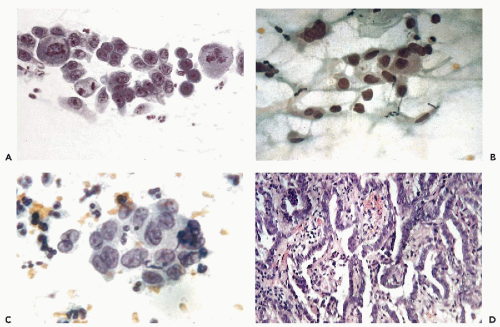
Large, complex clusters of tumor cells are often removed from the fragile surface of the tumor. Sometimes capillary vessels can be seen coursing through the cluster (Fig. 12-6A). Again, the search for columnar shape of the component cells and gland formation
within the cluster are the essential prerequisites of diagnosis.
Isolated “naked” nuclei of malignant cells are more common in brush than in scrape specimens and may be quite numerous (Fig. 12-6B). Some of these nuclei may appear quite bland and pale (Fig. 12-6C).
“Feathering” of cells on the surface of the cluster is less common, although this feature has been strongly emphasized in the literature. The term, introduced by Ayer et al (1987), pertains to clusters, wherein the peripheral cancer cells are approximately perpendicular to the long axis of the cluster, thus having some resemblance to a feather (Fig. 12-7B,C). It is essential to verify that the nuclear features of the component cells are those of a malignant tumor because, on rare occasions, this cell arrangement may also occur with benign endocervical cells and cells from other organs, such as the bronchus (Fig. 12-7D).
It is of historic interest that, in adenocarcinoma of the endocervix, the malignant cells will be found primarily in the direct cervical sample, whereas those of endometrial (and tubal or ovarian) origin will be found primarily in the vaginal pool smears which, nowadays, are unfortunately very rarely obtained. These differences cannot be recognized in liquid preparations.
Poorly Differentiated Endocervical Adenocarcinomas
Histology
These tumors are usually observed in advanced stages of disease as grossly visible tumors of the cervix. Their derivation from either the endocervical or endometrioid adenocarcinomas or, for that matter, poorly differentiated squamous (epidermoid) type of cancer may be difficult to determine. The tumor cells grow in solid sheets, wherein only occasional gland formation may be observed (Fig. 12-7).
Cytology
Poorly differentiated adenocarcinomas may be difficult to recognize because of necrotic debris and blood that are usually present in such preparations and may obscure cancer cells. The cancer cells derived from such tumors may retain some of the features of a well-differentiated carcinoma, notably the columnar shape of the cells and the spherical clustering. In many such cases, however, the cancer cells are of spherical or irregular configuration, vary in size, and have the characteristic nuclear features of advanced cancer, to wit, irregular shape, coarse chromatin arrangement and large nucleoli. In some cases, however, hyperchromasia may be absent and the large nuclei may be pale (see Fig. 12-6C). The cytoplasm is scanty and, therefore, the nucleocytoplasmic ratio is modified in favor of the nucleus. Abnormal mitotic figures may be observed. In such cases, the diagnosis of cancer is usually evident but the precise cytologic diagnosis of an adenocarcinoma may be difficult to establish and usually requires histologic evidence.
Microinvasive Adenocarcinoma
Histology
Using criteria applicable to the definition of microinvasive squamous carcinoma, Christopherson et al (1979) were apparently the first to apply this concept to cervical adenocarcinoma. Tumors infiltrating the cervical stroma to the depth of 5 mm or less were designated as microinvasive endocervical adenocarcinomas. The term was used by Bousfield et al (1980) in a large series of cases without further definition. Betsill and Clark (1986) defined this entity as tumors infiltrating to the depth of 2 mm or less. Mulvany and Östör (1997) described 24 cases classified as microinvasive adenocarcinoma with invasion of 5 mm or less. In such cases, small malignant glands or solid nests of cancer cells are seen in the cervical stroma, outside of the normal boundaries of the cancerous surface epithelium or glands. However, as previously discussed, the depth of distribution of normal endocervical glands varies from patient to patient and so do the boundaries. Such tumors virtually never form metastases.
It is our judgment that the diagnosis of microinvasive endocervical carcinoma is very difficult to establish, is not reproducible, and, in any event, it is of questionable practical value, because the prognosis of surgically treated endocervical microinvasive adenocarcinoma appears to be as favorable as that of carcinoma in situ, as documented by Betsill and Clark (1986), Östör et al (1997), and Mulvany and Östör (1997).
Cytology
Despite elaborate descriptions by Bousfield et al (1980) and Ayer et al (1988) from the same laboratory, it is doubtful that the cytologic identification of microinvasive carcinoma is possible in a reliable and reproducible fashion. In 12 of the 40 cases so classified, Mulvany and Östör (1997) observed more pleomorphic nuclei, coarse chromatin pattern, karyorrhexis and cell detritus and, hence, findings consistent with invasive adenocarcinoma described above.
PRECURSOR LESIONS OF INVASIVE ENDOCERVICAL ADENOCARCINOMA
Endocervical Adenocarcinoma In Situ
It is logical to expect that invasive endocervical adenocarcinomas are preceded by a cancerous change in the endocervical epithelium and glands. By definition, adenocarcinoma in situ is a malignant transformation of surface and endocervical gland epithelium in its normal anatomic setting. There are major individual differences in the distribution of endocervical glands and, in some women, normal glands may be found in the depth of the cervical stroma. When such deeply seated glands show malignant changes, it is sometimes difficult to state with certainty whether an adenocarcinoma is still in situ or whether an invasion has taken place. In most such cases, it is prudent to err on the conservative side.
Histology
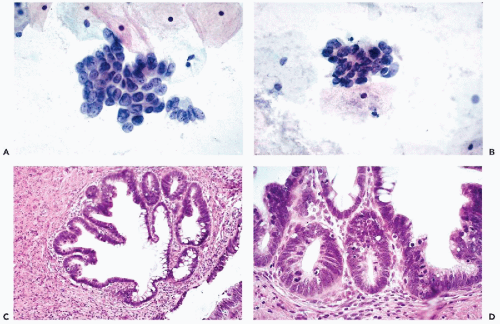
The affected epithelium lining the endocervical canal and the adjacent endocervical glands is composed of columnar, less often cuboidal, cancer cells that are usually larger than normal endocervical cells (Figs. 12-8C,D, 12-9D, 12-10C,D). The size and configuration of the cancer cells is best assessed in cases wherein there is a clear-cut transition of normal to cancerous epithelium within the same or adjacent glands (Figs. 12-9D and 12-10D). In some of the glands, “bridges” of cancer cells criss-crossing the lumen may be observed (Fig. 12-9D), as is also the case in other ductal carcinomas such as the breast (see Chap. 29). The epithelium of some adenocarcinomas in situ contains Paneth cells with cytoplasmic granules, suggestive of intestinal differentiation. Although the malignant epithelium may be formed by a single, fairly orderly layer of cuboidal or columnar cells, similar to the normal endocervix, in many areas the cancer cells form two or three layers and short papillary projections. Nuclear crowding may be particularly evident at the tips of the papillae.
In the cancerous epithelium, the nuclei are enlarged, of irregular contour, hyperchromatic, and sometimes coarsely granular. In some cells, readily visible nucleoli are present but this is rarely the dominant feature of these cells. Mitotic figures are often evident in the cancerous epithelium and are an important diagnostic feature because mitoses are rare in normal endocervical epithelium (Fig. 12-8D). Biscotti and Hart (1998) pointed out that
the presence of apoptotic bodies (i.e., nuclear necrosis with coarse fragmentation of chromatin; see Chap. 6), within the malignant epithelium, is characteristic of this disorder.
the presence of apoptotic bodies (i.e., nuclear necrosis with coarse fragmentation of chromatin; see Chap. 6), within the malignant epithelium, is characteristic of this disorder.
The configuration of carcinoma in situ is similar in nearly all cases, although Jaworski et al (1988) classified two cases as “endometrioid carcinoma in situ,” because of absence of mucus production in the lesions. In a large proportion of cases, endocervical adenocarcinomas in situ are accompanied by preinvasive lesions of squamous type (CIN) or even invasive squamous carcinoma (see below).
Natural History
There are few reports in the literature about progression of endocervical carcinoma in situ to invasive carcinoma. In the case reported by Büttner and Kyank (1973), 11 years elapsed before invasive carcinoma developed. Boddington et al (1976) reviewed prior cervical smears in 13 women who developed endocervical adenocarcinoma. In six of these patients, prior cytologic abnormalities were observed over a period of several years, leading to the conclusion that adenocarcinomas have an evolution extending over two to eight years. On retrospective review, Boon et al (1981) observed adenocarcinoma in situ that was not recognized in biopsies obtained three to seven years before the development of invasive adenocarcinoma. Lee and Flynn (2000) estimated that the progression of adenocarcinoma in situ to invasive cancer requires approximately 5 years.
Personal observations also point to a long developmental period in the natural history of these lesions. This information is anecdotal, based generally on cases in which a cytologic diagnosis of adenocarcinoma was followed by endocervical curettage in which evidence of adenocarcinoma was not recognized, some years prior to the development of invasive tumor. The most common source of biopsy error was the presence of strips of mucus-forming epithelium which, in spite of nuclear abnormalities, were thought to represent benign endocervical epithelium (see Fig. 12-11). Because of the life-threatening nature of endocervical adenocarcinoma, no follow-up studies of untreated adenocarcinoma in situ have been conducted and none should be contemplated. However, in follow-up studies of several personally observed patients treated for adenocarcinoma in situ by conization, it was noted that these patients were prone to the development of other neoplastic events in the cervix in the form of recurrent adenocarcinoma or squamous precursor lesions (CIN).
Cytology
Adenocarcinoma in situ of the cervix has been recognized in the Bethesda System 2001 as a separate cytologic category.
The cytologic presentation of this entity has been extensively discussed in the literature (Qizilbash, 1975; Betsill and Clark, 1986; Ayer et al, 1987



The cytologic presentation of this entity has been extensively discussed in the literature (Qizilbash, 1975; Betsill and Clark, 1986; Ayer et al, 1987
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree