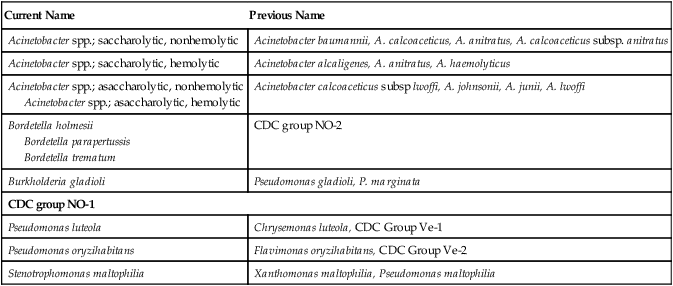
1. List the most common gram-negative organisms discussed in this chapter that are encountered in clinical specimens. 2. Explain where Acinetobacter spp. are found and the patients most at risk of infection. 3. Describe the Gram stain morphology of Acinetobacter, Bordetella, and Stenotrophomonas spp. 4. Describe the appearance and odor of Stenotrophomonas maltophilia when grown on blood agar. 5. Differentiate between the two groups of Acinetobacter organisms and identify the most dependable test to distinguish between the groups. The organisms discussed in this chapter are considered together because, except for CDC group NO-1, they are all oxidase negative and grow on MacConkey agar, as do the Enterobacteriaceae. However, unlike the Enterobacteriaceae, which ferment glucose, these organisms either oxidize glucose (i.e., they are saccharolytic), or they do not utilize glucose (i.e., they are nonoxidizers, or asaccharolytic). Although CDC group NO-1 is oxidase negative and does not usually grow on MacConkey agar, it is included here because it must be distinguished from the asaccharolytic Acinetobacter spp. Based on molecular studies, approximately 21 species and/or strains of Acinetobacter spp. have been identified. The specific morphologic and physiologic features of the organisms are considered later in this chapter in the discussion of laboratory diagnosis. Of note, only Acinetobacter and Stenotrophomonas spp. are routinely found in clinical specimens. Bordetella parapertussis is included in Table 21-4 in this chapter but is discussed in Chapter 37. The organisms discussed in this chapter inhabit environmental niches. Acinetobacter spp. and Stenotrophomonas maltophilia are widely distributed in moist natural and hospital environments (Table 21-1). Acinetobacter spp. can be found on fomites and in soil, water, and animal food products. These organisms are capable of survival on inanimate objects for extended periods. Acinetobacter spp. is a human skin colonizer in 0.5% to 3% of the general population and has been identified from a number of human sources, including sputum, urine, feces, and vaginal secretions. S. maltophilia may be found in tap water and salads. Although none of these organisms are considered normal human flora, the relatively high prevalence of Acinetobacter spp. and S. maltophilia in hospitals frequently results in colonization of the skin and respiratory tract of patients. The prevalence of these organisms is evidenced by the fact that, excluding the Enterobacteriaceae, Acinetobacter spp. and S. maltophilia are the second and third most common gram-negative bacilli, respectively, encountered in clinical specimens. In contrast, Pseudomonas luteola, Pseudomonas oryzihabitans, and CDC group NO-1 are not commonly found in clinical specimens but have been isolated from wounds, blood cultures, and dialysis fluids. TABLE 21-1 All of the organisms listed in Table 21-2 are opportunistic pathogens for which no definitive virulence factors are known. Because Acinetobacter spp. and S. maltophilia are relatively common colonizers of hospitalized patients, their clinical significance when found in patient specimens can be difficult to establish. In fact, these organisms are more frequently isolated as colonizers than as infecting agents. When infection does occur, it usually is seen in debilitated patients, such as those in burn or intensive care units and those who have undergone medical instrumentation and/or have received multiple antimicrobial agents. Acinetobacter baumannii is typically the species identified in hospital-acquired infections. Infections caused by Acinetobacter spp. and S. maltophilia usually involve the respiratory or genitourinary tract, bacteremia and, occasionally, wound infections, although infections involving several other body sites have been described. Community-acquired infections with these organisms can occur, but the vast majority of infections are nosocomial. TABLE 21-2 Pathogenesis and Spectrum of Diseases No special considerations are required for specimen collection and transport of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport. Table 21-3 describes the colonial appearance and other distinguishing characteristics (e.g., hemolysis and odor) of each genus when grown on 5% sheep blood and MacConkey agars. TABLE 21-3 Colonial Appearance and Characteristics
Acinetobacter, Stenotrophomonas, and Similar Organisms
General Characteristics
Epidemiology
Species
Habitat (Reservoir)
Mode of Transmission
Acinetobacter spp.
Widely distributed in nature, including the hospital environment. May become established as part of skin and respiratory flora of patients hospitalized for prolonged periods
Colonization of hospitalized patients from environmental factors; medical instrumentation (e.g., intravenous or urinary catheters) introduces organism to normally sterile sites
Stenotrophomonas maltophilia
Widely distributed in nature, including moist hospital environments. May become established as part of respiratory flora of patients hospitalized for prolonged periods
Colonization of hospitalized patients from environmental factors; medical instrumentation introduces organism to normally sterile sites (similar to transmission of Acinetobacter spp.)
CDC group NO-1
Oropharynx of animals. Not part of human flora
Animal bite or scratch
Burkholderia gladioli
Environmental pathogen of plants; occasionally found in respiratory tract of patients with cystic fibrosis but not part of normal flora
Transmission to humans uncommon, mode of transmission not known
Pseudomonas luteola
Pseudomonas oryzihabitans
Environmental, including moist hospital environments (e.g., respiratory therapy equipment). Not part of normal human flora
Uncertain; probably involves exposure of debilitated hospital patients to contaminated fluids and medical equipment
Bordetella holmesii
B. trematum
Unknown or part of normal human flora
Unknown; rarely found in humans
Pathogenesis and Spectrum of Disease
Species
Virulence Factors
Spectrum of Disease and Infections
Acinetobacter spp.
Unknown
Clinical isolates are often colonizers. True infections are usually nosocomial, occur during warm seasons, and most commonly involve the genitourinary tract, respiratory tract, wounds, soft tissues, and bacteremia
Bordetella holmesii
Bordetella trematum
Unknown
Bacteremia is the only type of infection described.
Burkholderia gladioli
Unknown
Role in human disease is uncertain; occasionally found in sputa of patients with cystic fibrosis, but clinical significance in this setting is uncertain.
Pseudomonas luteola, P. oryzihabitans
Unknown
Catheter-related infections, septicemia, and peritonitis, usually associated with continuous ambulatory peritoneal dialysis, and miscellaneous mixed infections of other body sites.
Stenotrophomonas maltophilia
Unknown. Intrinsic resistance to almost every commonly used antibacterial agent supports the survival of this organism in the hospital environment.
Most infections are nosocomial and include catheter-related infections, bacteremia, wound infections, pneumonia, urinary tract infections, and miscellaneous infections of other body sites.
CDC group NO-1
Unknown
Animal bite wound infections
Laboratory Diagnosis
Specimen Collection and Transport
Cultivation
Media of Choice
Colonial Appearance
Organism
Medium
Appearance
Stenotrophomonas maltophilia
BA
Large, smooth, glistening colonies with uneven edges and lavender-green to light purple pigment; greenish discoloration underneath growth; ammonia smell
Mac
NLF
Acinetobacter spp.
BA
Smooth, opaque, raised, creamy, and smaller than Enterobacteriaceae; some genospecies are beta-hemolytic
Mac
NLF, but colonies exhibit a purplish hue that may cause the organism to be mistaken for LF (Figure 21-1)
Burkholderia gladioli
BA
Yellow
Mac
NLF
Bordetella parapertussis
BA
Smooth, opaque, beta-hemolytic
Mac
NLF, delayed growth
Bordetella holmesii
BA
Punctate, semiopaque, convex, round, with greening of blood usually accompanied by lysis
Mac
NLF, delayed growth
Bordetella trematum
BA
Convex, circular, grayish cream to white
Mac
NLF
Pseudomonas oryzihabitans
BA
Wrinkled, rough or smooth, transparent, yellow
Mac
NLF
Pseudomonas luteola
BA
Maybe rough and smooth, opaque, yellow
Mac
NLF
CDC group NO-1
BA
Small colonies that can be transferred intact with an inoculating needle
Mac
NLF, but only 20% of strains grow ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree