Chapter 5 Acantholytic disorders
Introduction
The term acantholysis derives from the Greek akantha, a thorn or prickle, and lysis, a loosening. In its simplest definition, the term is used to reflect a primary disorder of the skin (and sometimes the mucous membranes) characterized by separation of the keratinocytes at their desmosomal junctions (Fig. 5.1). A wide range of conditions are characterized by this feature, from inherited disorders such as Darier’s disease and Hailey-Hailey disease in which a calcium pump gene mutation results in desmosomal instability through to the autoimmune pemphigus group of diseases whereby autoantibodies directly damage desmosomes with resultant keratinocyte separation and blister formation (Table 5.1). Desmosomes may also be damaged by secondary phenomena, for example following severe edema, either intercellular (spongiosis) or intracellular (e.g., ballooning degeneration as is seen in various viral infections). Such processes, however, are not included in the acantholytic category and are discussed elsewhere. The histological features of the conditions described in this chapter show considerable overlap. The diagnosis is therefore dependent upon adequate clinical information and the results of immunofluorescence investigations.
Table 5.1 Antigens targeted in the pemphigus variants
| Pemphigus variant | Autoantigen |
|---|---|
| Pemphigus vulgaris | Dsg3 (mucosal), Dsg1 (cutaneous), desmocollins, pemphaxin, α9-acetylcholine receptor |
| Pemphigus vegetans | Dsg3, Dsc1, and Dsc2 in some patients |
| Pemphigus foliaceus | Dsg1 |
| Pemphigus erythematosus | Dsg1 |
| Fogo selvagem | Dsg1, rarely also Dsg3 |
| IgA pemphigus | Dsc1, Dsg1 or Dsg3 |
| Herpetiform pemphigus | Dsg1, rarely also Dsg3 |
| Paraneoplastic pemphigus | Desmoplakins I and II, envoplakin, periplakin, BP230, plectin, Dsg1, and Dsg3 |
| Drug-induced pemphigus | Dsg1 or Dsg3 |
Dsc, desmocollin; Dsg, desmoglein. Modified from Martel, P., Joly, P. (2001) Pemphigus: autoimmune diseases of keratinocyte’s adhesion molecules. Clinical Dermatology, 19, 667.
Pemphigus
Pemphigus (Gr. pemphix, blister) refers to a group of chronic blistering diseases which develop as a consequence of autoantibodies directed against a variety of desmosomal proteins.1–5 The condition as a whole is rare, with an annual incidence ranging from 0.1–0.7 per 100 000 of the general population.2 It is commoner in the Jewish population in which the annual incidence rises to 1.6–3.2 per 100 000.6 Ashkenazi Jews are the most frequently affected.6 The incidence in India also appears to be higher than in other countries.7 There is no sex predilection.
Pemphigus vulgaris is by far the most common variant, accounting for 80% of cases.8,9
In addition to affecting humans, pemphigus has been described in a variety of animals including dogs, cats, goats, and horses.10
Pemphigus vulgaris
Clinical features
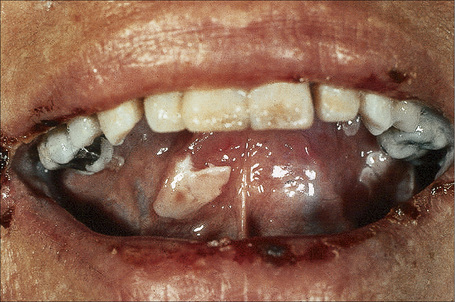
Pemphigus vulgaris (p. vulgaris) particularly affects the middle aged (onset typically at 40–60 years of age) although occasionally (up to 2.6%) children are affected.1–7 Self-limiting neonatal disease through transplacental transfer of maternal autoantibodies has also rarely been documented (see pathogenesis).8–11 The disease begins in the mouth (Figs 5.2, 5.3) in 50–70% of patients with painful erosions or bullae and, after a period of weeks or months, the blisters spread to involve the skin.12–15 Oral lesions most commonly affect the buccal, palatine, and gingival mucosae.1,15–17 Pemphigus vulgaris is only rarely confined to the skin.18,19
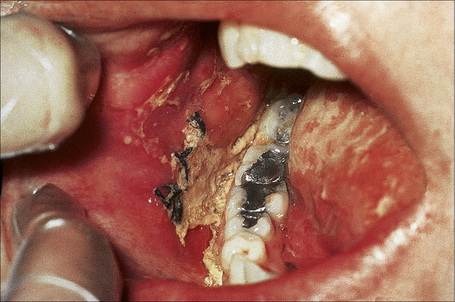
Fig. 5.2 Pemphigus vulgaris: painful erosions are present on the buccal mucosa.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
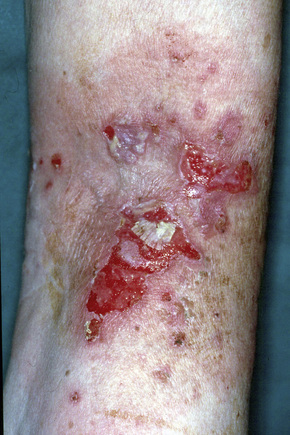
The typical skin lesion is a fragile, flaccid blister, which develops on normal or erythematous skin, and readily ruptures, leaving a painful, crusted, raw, bloody erosion (Figs 5.4, 5.5). Lesions are most often seen on the scalp, face, axillae, and groin, although in some patients they are generalized (Figs 5.6–5.8).1–3,20 Blisters can be induced by rubbing the adjacent, apparently normal skin with a finger – the Nikolsky sign. Direct pressure applied to the center of the blister is also followed by lateral extension – the Asboe-Hansen sign.2 Healing is often accompanied by postinflammatory hyperpigmentation but scarring is not a feature.2
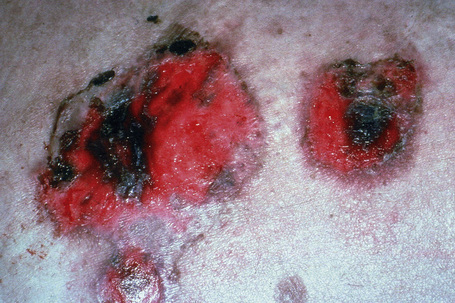
Fig. 5.4 Pemphigus vulgaris: since the blisters are superficial, erosions are more commonly encountered.
By courtesy of the Institute of Dermatology, London, UK.
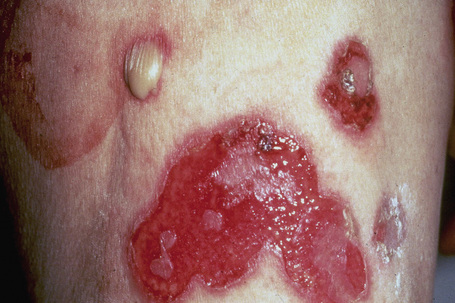
Fig. 5.5 Pemphigus vulgaris: extensive erosions and blisters are present on the shin.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
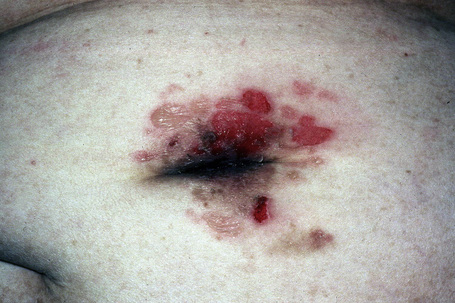
Fig. 5.6 Pemphigus vulgaris: umbilical lesions showing intact blisters as well as raw erosions.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
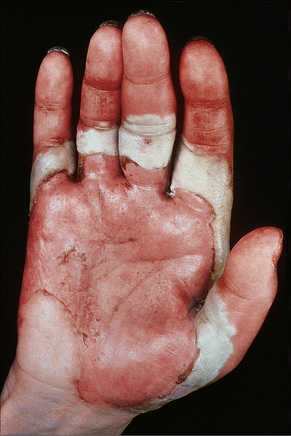
Fig. 5.7 Pemphigus vulgaris: extensive trauma-induced blisters.
By courtesy of the Institute of Dermatology, London, UK.
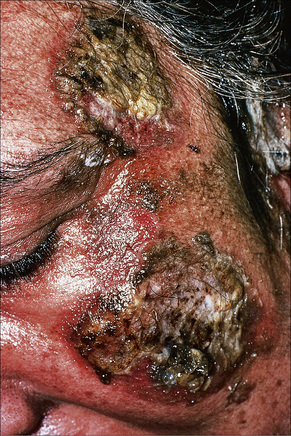
Fig. 5.8 Pemphigus vulgaris: extensive disease can be very disfiguring.
By courtesy of the Institute of Dermatology, London, UK.
Before the introduction of corticosteroid therapy, the lesions usually became more extensive and in the past often led eventually to death. Treatment with high doses of corticosteroids, immunosuppressants, such as azathioprine and more recently biologicals has significantly reduced the mortality to 5–15% and prolonged remissions without treatment are now being reported.2 A considerable proportion of the deaths that do occur, however, are due to the side effects of therapy and include staphylococcal infections and, to a lesser extent, pulmonary embolism.2 Severe opportunistic infections due to a wide range of organisms including listeria, nocardia, enterococci, herpes virus, cryptococcus and candida may further complicate the disease.21–27
Nail involvement seems to be more common than previously reported. Patients may present with hemorrhagic paronychia, chronic paronychia, trachyonychia, onycholysis or onychomadesis.17,28 Paronychia and onychomadesis are the most common nail changes encountered. Nail involvement is more common in the nails of digits affected by periungual blisters and also in patients with large number of skin blisters.29
Occasional modes of presentation include linear lesions and pemphigus arising after surgery, burns, vaccination, and radiation therapy.30–39 Development after exposure to pesticides and a possible association with cocaine snorting has also been reported.40,41 A very exceptional case has been described in which blisters were initially confined to melanocytic nevi.42 P. vulgaris may also be rarely induced by a variety of drugs.
In addition to oral and cutaneous involvement, lesions have been described at a wide variety of sites including the nasopharynx, larynx, ear, esophagus, eye, external genitalia, urethra, and anal and colonic mucosa.1,43–45 Esophageal lesions, although originally thought to be rare, have more recently been documented in as many as 63–87% of patients.46,47 Erosions and ulcers are typically found and intact blisters are rare. Exceptionally, the whole mucosa may be affected with subsequent sloughing – esophagitis dissecans superficialis.48 Ocular lesions are usually restricted to the conjunctiva, presenting as conjunctivitis or small vesicles that rapidly rupture.2,49,50 Very rarely, scarring may develop and corneal ulceration with perforation has been described.51 Vulval, vaginal, and cervical lesions are well recognized.52–56 Exceptionally, the vagina may be the sole site of involvement.57 Penile lesions most commonly affect the glans.58 They are not usually followed by any significant sequelae.
The development of pemphigus may be associated with a variety of disorders including other autoimmune bullous dermatoses, particularly bullous pemphigoid, lupus erythematosus, thymoma, and myasthenia gravis as well as Hashimoto’s thyroiditis, vitiligo, minimal change nephropathy, and ulcerative colitis.59–66 It has also been described in a patient with the 1p36 deletion syndrome.67 As in the many other diseases with an immunological pathogenesis, pemphigus is accompanied by an increased incidence of internal malignancy including thymoma, lymphoma, and multiple myeloma (see paraneoplastic pemphigus).68,69 It has also been reported in association with Kaposi’s sarcoma.70
Pathogenesis and histological features
Pemphigus is an immunologically mediated disease.71,72 Examination of perilesional skin by direct immunofluorescent techniques reveals in vivo-bound immunoglobulin (usually IgG) and often complement (C3) in the intercellular region of the epidermis (Fig. 5.9).73 Abundant antigen in the follicular outer root sheath and germinal matrix may account for the marked scalp involvement typical of pemphigus, and plucked hair follicles may serve as an adequate substrate for direct immunofluorescence analysis.74,75 The in vivo-bound IgG is mainly of the IgG1 and IgG4 subclasses.76
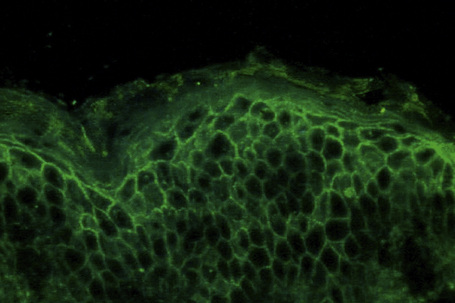
Fig. 5.9 Pemphigus vulgaris: direct immunofluorescence.
By courtesy of the Institute of Dermatology, London, UK.
Indirect immunofluorescent techniques show that the serum of patients with pemphigus contains an IgG antibody that reacts with the intercellular region of normal squamous epithelium – the intercellular substance (pemphigus) antibody.77 This antibody is, however, not entirely specific as it may be found in a variety of other conditions, such as severe burns, penicillin drug reactions, and following radiation therapy.78–80 Presumably, pemphigus antigens are released into the circulation following such trauma with resultant antibody production. Circulating antibodies are predominantly of the IgG1 and IgG4 subclasses; IgG3 is identified much less often.81
Circulating IgG is pathogenic.71,72 The level of the antibody titer closely parallels the clinical state of the disease.82–85 IgG4 titers diminish during remission whereas circulating IgG1 may continue to be present.72,83 Relapse is commonly preceded by rising IgG4 antibody titers.83
P. vulgaris very occasionally may be evident in a neonate born of a mother with active pemphigus vulgaris.8,86 Such autoantibodies cross the placenta, inducing disease in the infant. The condition is, however, short lived, with lesions disappearing, as the maternal antibodies are catabolized. Passive transfer of IgG4 into neonatal mice results in the development of blisters.87 Purified IgG from pemphigus induces acantholysis in human skin explants and keratinocyte cultures.88,89
The pemphigus antibody binds to the full thickness of the epidermis. Compared with p. vulgaris, immunofluorescence studies on the sera of p. foliaceus patients tend to show more staining in the superficial epidermis, correlating with the level of the split.90,91 Conversely, the sera from patients with p. vulgaris show more affinity for the lower epidermis. Despite these trends, we generally do not base diagnoses on these (often subtle) differences in immunofluorescence staining distribution.
The p. vulgaris antibody is directed at the extracytoplasmic domain of the 130-kD epithelial desmosomal cadherin, desmoglein 3 (Dsg3), which forms a complex with plakoglobin (85 kD).92–98 The p. vulgaris antibody, however, does not recognize the latter. Many patients also have antibodies that bind to the p. foliaceus antigen, desmoglein 1 (Dsg1), a 160-kD polypeptide.99,100 Dsg3 is expressed primarily in the oral mucosa and, therefore, antibodies directed against this antigen result in mucosal pemphigus. In contrast, Dsg1 is a cutaneous antigen and, therefore, antibodies directed against it result in lesions affecting the skin but not the mucosa (cutaneous pemphigus).90 Anti-Dsg1 antibodies also show cross-reactivity against Dsg4, a recently identified member of the desmoglein family.101,102 While patient sera contain antibodies against nonconformational epitopes of Dsg3, active disease correlates with the presence of antibodies directed against the NH2-terminal aspect of Dsg3, in particular ectodomains 2–4.103–105 Oral disease is particularly associated with reactivity to ectodomains 1-4, which is reduced in cutaneous pemphigus.103
Antibodies reactive to a number of other proteins including desmoplakin, desmocollins, pemphaxin, and acetylcholine receptor have also been demonstrated in the sera of p. vulgaris patients.106–110
Sera from patients with pemphigus vulgaris not infrequently contain additional IgA antibodies, in particular against Dsg1 and Dsg3.111–113 Although the combination of both IgG and IgA antibodies has in some instances been referred to as IgG/IgA pemphigus in the literature, this appears to be an ill-defined and heterogeneous disease group.111,114 In addition to pemphigus vulgaris, the additional presence of anti-Dsg IgA antibodies has also been demonstrated in pemphigus foliaceus, pemphigus vegetans, pemphigus herpetiformis, and paraneoplastic pemphigus.111,115,116 Furthermore, so-called IgG/IgA pemphigus may show an atypical clinical presentation, histological features more reminiscent of IgA pemphigus, and the presence of IgA antibodies against desmocollins in a subset of patients.114,117–123
The pathogenesis of the acantholysis is uncertain. Direct binding of antibody to the desmosomal cadherins is of major importance and results in internalization of Dsg3 and degradation by the endolysosomal pathway.71,114,124 Plakoglobin has been implicated in mediating intracellular events following IgG binding to Dsg3.125,126 In particular, the role of plakoglobin is signal transduction to the nucleus.125,127 There is also some evidence to suggest that the process may involve, at least secondarily, the action of local proteolytic enzymes.70 Pemphigus antibody induces expression of plasminogen activator receptor on the surface of keratinocytes.128 Binding of plasminogen activator to its keratinocyte cell membrane receptor results in plasminogen activation with resultant production of plasmin.129,130 This latter has non-specific proteolytic activity, which may be responsible at least in part for the dissolution of the desmosomes.71 P. vulgaris antibodies stimulate production of keratinocyte phospholipase C, inositol 1,4,5-triphosphate, and increase intracellular calcium. Protein kinase C activation results in release of keratinocyte plasminogen activator and increased expression of plasminogen activator receptor.131–133 Other factors, however, must be of greater importance since p. vulgaris IgG can induce acantholysis in plasminogen activator knockout mice.134 An additional phenomenon is rapid phosphorylation of heat shock protein 27 and p38MAPK resulting in reorganization and collapse of the cytoskeleton as a result of IgG binding to Dsg3.135,136 This process is mediated by upstream events involving EGF receptor kinase and src.137 Complement appears not to be essential for acantholysis and it is thought that any involvement is secondary, perhaps accelerating or extending the process.71 Although it has been suggested that apoptosis may be induced by p. vulgaris IgG, and that this mechanism may be important in the pathogenesis of the disease, a recent study has shown that apoptosis is not a prerequisite for blistering and may be a secondary phenomenon.138
T cells are also critical to the development of the antibody-mediated acantholysis.70 CD4+ memory T cells are predominantly involved and both T-helper 1 (Th1) and Th2 Dsg3-specific subtypes are represented.139,140 Th1 T-cell-derived interferon-γ stimulates production of IgG1, and Th2 cells produce interleukin (IL)-4 and IL-13 which are responsible for secretion of B-cell-derived IgG4.141 Both populations are therefore of importance in stimulating production of p. vulgaris antibody.72 In addition, there is evidence that tumor necrosis factor 1 (TNF-1), Fas-ligand and IL-1 are also of importance in the development of acantholysis.142 Knockout mice for both these cytokines show diminished acantholysis in passive antibody transfer experiments.143
There is considerable evidence of a genetic background influencing susceptibility to pemphigus as shown by strong associations with human leukocyte antigen (HLA)-DRβ1*0402, HLA-DRβ1*1401 and HLA-DQβ1*0503.144–147 Perhaps surprisingly, however, there are only occasional documented reports of familial occurrence.148–151
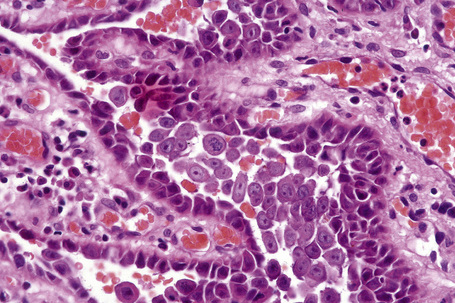
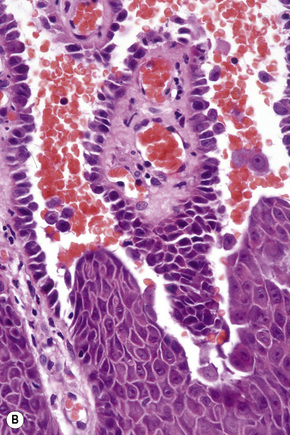
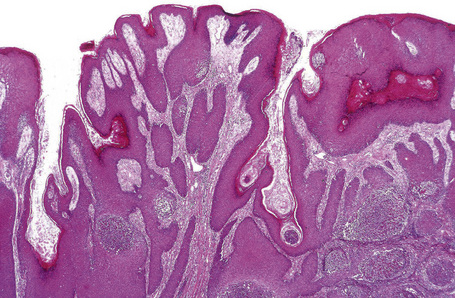
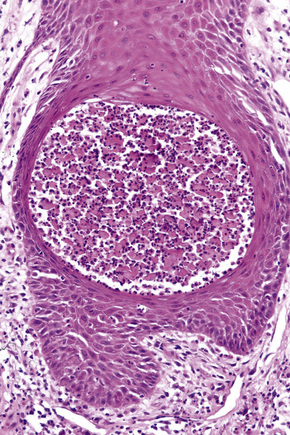
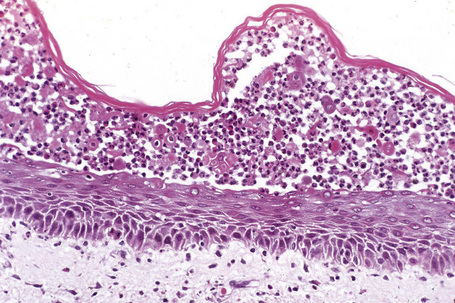
Pemphigus blisters rupture easily. It is therefore essential to biopsy an early lesion to establish the correct diagnosis. The characteristic acantholysis develops because of damage to the intercellular bridges. Acantholytic cells are rounded and have intensely eosinophilic cytoplasm, pyknotic nuclei, and perinuclear halos. An early lesion of p. vulgaris shows a slitlike suprabasal cleft or vesicle containing occasional acantholytic cells. The established blister contains acantholytic cells in clumps and in isolation (Figs 5.10 and 5.11). Characteristically, the floor of the cavity is lined by a single layer of intact basal cells, the so-called ‘tombstone’ pattern (Fig. 5.12).152
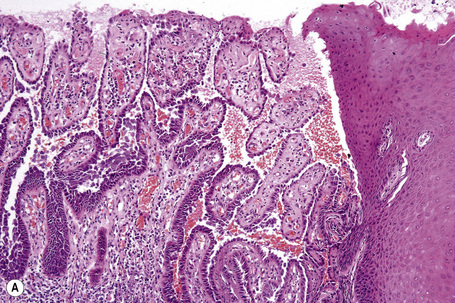
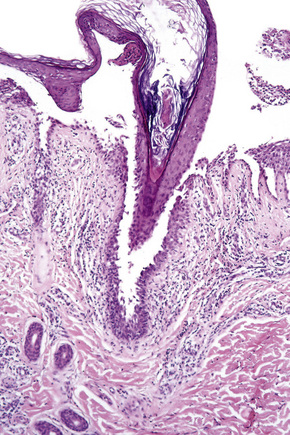
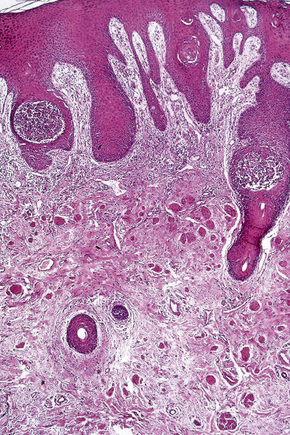
The acantholytic process frequently involves the epithelium of the adnexae, which can be a useful diagnostic clue in those lesions which lack the roof of the blister (Fig. 5.13).153 The dermal papillary outline is usually maintained and, frequently, the papillae protrude into the blister cavity. Sometimes the features of eosinophilic spongiosis are seen on biopsy, particularly in early lesions.154 The blister cavity often contains a few inflammatory cells (notably eosinophils) and, in the dermis, there is a moderate perivascular chronic inflammatory cell infiltrate with conspicuous eosinophils, although sometimes these are scanty or even absent. Mucous membrane lesions show similar histology.
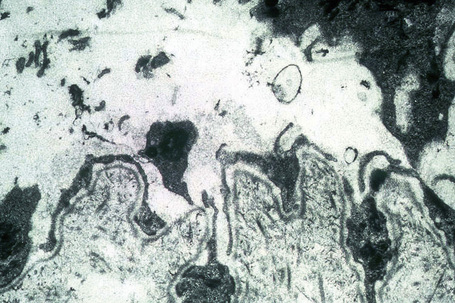
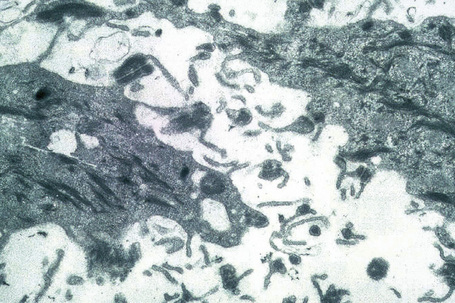
Ultrastructurally, there is dilatation of the intercellular space with consequent stretching of the desmosomal attachment points (Figs 5.14, 5.15).155 With progression, these separate and eventually disappear, residual cell membranes often showing a pseudovillous morphology. Hemidesmosomes are morphologically normal. Immunoelectron microscopy confirms that the immunoreactants are located within the intercellular space.
Endemic pemphigus vulgaris
Patients with clinical and histological presentation of pemphigus vulgaris but epidemiological features of fogo selvagem were identified in the Goiania and Brasilia regions of Brazil, known endemic areas of pemphigus foliaceus. These patients demonstrate classical mucocutaneous disease and antibodies to both Dsg1 and Dsg3, but are remarkable for early onset of disease, frequently before the age of 20.156
Differential diagnosis
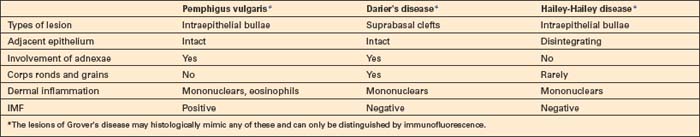
The differential diagnosis of p. vulgaris includes a variety of conditions such as Darier’s disease, Hailey-Hailey disease, and transient acantholytic dermatosis (Grover’s disease) (Table 5.2). In the absence of clinical information or without immunofluorescence studies, it may be impossible to establish a definitive diagnosis. Darier’s and Hailey-Hailey diseases are not associated with immunoreactants.
Pemphigus vegetans
Clinical features
Pemphigus vegetans (p. vegetans), a chronic variant of p. vulgaris, has a somewhat better prognosis than p. vulgaris with occasional cases associated with spontaneous remission documented.1–3 It accounts for 1–2% of all cases of pemphigus.1 As with the vulgaris variant, p. vegetans typically presents in adults. There has, however, been a small number of cases described in childhood including a dapsone-responsive IgA-mediated variant.4–7 The lesions, which present as blisters and erosions, are particularly prolific in the flexures, especially the axillae, the groin, the inframammary region, the umbilicus and at the margins of the lips. The scalp is also said to be a site of predilection.8,9 Soon thereafter, patients characteristically develop hypertrophic vegetations and pustules at the blistered edges (Fig. 5.16).1
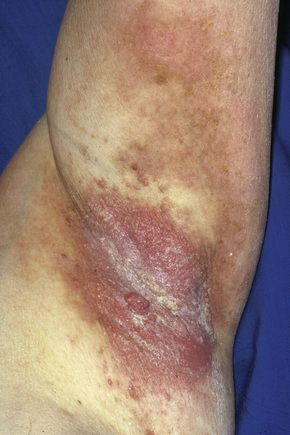
Fig. 5.16 Pemphigus vegetans: axillary ulceration and vegetative lesions.
From the slide collection of the late N.P. Smith, MD, The Institute of Dermatology, London, UK.
The oral cavity is commonly affected and a cerebriform or ‘scrotal’ tongue is said to be a diagnostic clue in cases of early involvement.10–13 An exceptional case of the disease restricted to the tongue has been reported.14 Esophageal involvement presenting as erosions and white plaques has been described in a number of patients and the nasal mucosa, larynx, vulva, vagina, penis, and anus may also be affected.7,15–19 Nail involvement including onycholysis and pustules is sometimes seen.20 Acral involvement can clinically be mistaken for acrodermatitis continua suppurativa.21 A case has been described developing after and restricted to a split-thickness skin graft.22 A further exceptional case developed in association with intranasal heroin abuse and was restricted to the nasal mucosa.23 Peripheral blood eosinophilia is commonly present.
Two clinical subtypes are recognized:24,25
Pathogenesis and histological features
Support for the thesis that p. vegetans is a variant of p. vulgaris is based on the finding that both subtypes are associated with IgG and C3 deposition in the epidermal intercellular space on direct immunofluorescence, and circulating ‘pemphigus’ antibody.25 P. vegetans is characterized by an antibody directed at the desmosomal cadherin, desmoglein 3.26–28 Antibodies against desmocollins 1 and 2 as well as periplakin have also been documented.29,30 Rarely, additional IgA antibodies to Dsg3 may also be detected.31
Precipitating factors for this variant of pemphigus are largely unknown. Exceptionally, however, p. vegetans has been linked with the angiotensin-converting enzyme (ACE) inhibitors, captopril and enalapril.32,33 Lesions localized to the nasal mucosa in a patient with longstanding nasal heroin abuse have been reported and an association with human immunodeficiency virus (HIV) infection documented.19,34,35 There are few reports relating p. vegetans with an underlying malignancy and in one patient a p.vegetans-like lesion was a manifestation of paraneoplastic pemphigus.33,36–40
Although a variant of p. vulgaris, p. vegetans shows strikingly different histological features. Suprabasal acantholysis is present but is often subtle, being masked by an exuberant proliferation of squamous epithelium which may sometimes show pseudoepitheliomatous hyperplasia (Fig. 5.17). The epithelial proliferation involves both the epidermis and the infundibular follicular epithelium. Characteristically, there is an intense inflammatory cell infiltrate containing numerous eosinophils, and intraepidermal microabscesses are often seen (Figs 5.18, 5.19). Eosinophilic spongiosis is a feature.41,42 The inflammatory changes and epithelial proliferation are sometimes so marked that the true nature of the lesions is obscured. Very occasionally, 10–40-μm eosinophilic hexagonal Charcot-Leyden crystals have been described within the eosinophil-rich microabscesses.32,43 The diagnosis of p. vegetans is easily overlooked and is made only by the pathologist with a high index of suspicion.
Pemphigus foliaceus
Clinical features
Pemphigus foliaceus (p. foliaceus) is considerably more uncommon than p. vulgaris and although it most often affects the middle aged and elderly, it has a very variable age of onset, sometimes affecting younger adults and even, occasionally, children.1–8 Very exceptionally, maternal antibodies have been known to cross the placenta, resulting in neonatal disease.9–11 In general, nonendemic p. foliaceus in children is relatively benign and of short duration.6
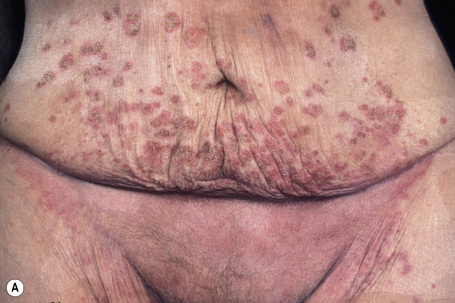
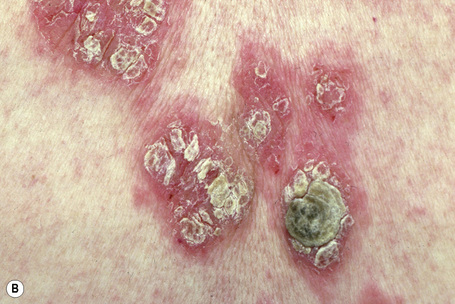
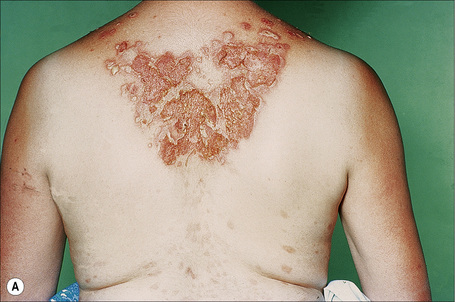
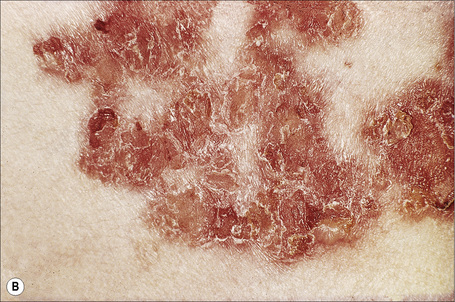
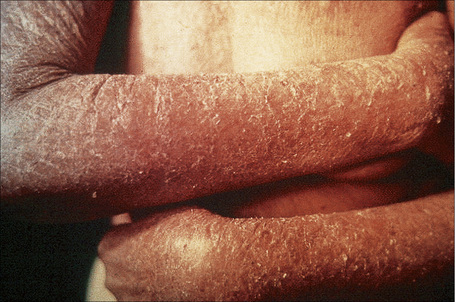
The superficial blisters of p. foliaceus are exceedingly fragile and there fore much less obvious; erosions and large leafy scales or crusts are often predominant (Figs 5.20–5.22). The lesions may remain localized to the scalp, nose, face, and trunk for many months or years, leading to a mistaken diagnosis of seborrheic dermatitis, seborrheic keratosis, or even lupus erythematosus. Sometimes the eruption involves the entire surface of the body or produces a clinical resemblance to exfoliative dermatitis (erythroderma) (Fig. 5.23).12,13 Mucous membrane involvement is rare.1 Exceptionally, patients may present with localized disease, typically restricted to the face.14,15 The development of pustular lesions is exceptional.16 P. foliaceus often has a much more benign course than p. vulgaris, although patients with severe disease, requiring corticosteroid and immunosuppressant therapy, still have an appreciable mortality. The disease may be complicated by Kaposi’s varicelliform eruption.17
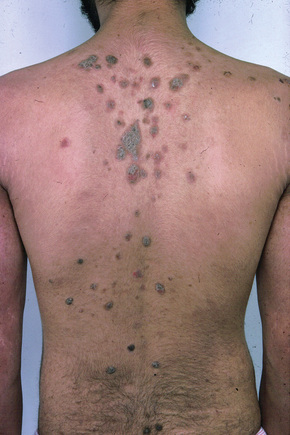
Fig. 5.21 Pemphigus foliaceus: crusted lesions are evident on the back of this young male.
From the collection of the late N.P. Smith, MD, The Institute of Dermatology, London, UK.
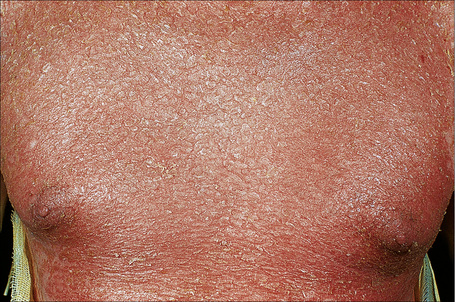
Fig. 5.23 Pemphigus foliaceus: in this patient, there is generalized erosion with scaling and erythroderma.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
Very occasionally, patients may develop p. foliaceus during or after a previous episode of p. vulgaris and vice versa.18–20 The development of bullous pemphigoid following an episode of p. foliaceus has also been described.21,22 This is accompanied by an antigen shift, possibly as a result of intermolecular epitope spreading.19,23–25 A case of a blistering disorder displaying features of bullous pemphigoid and pemphigus foliaceus has been described in association with consumption of Spirulina algae.26 The coexistence of both p. vulgaris and p. foliaceus in one patient has also been reported.27 A further case of paraneoplastic pemphigus with concomitant clinical features of pemphigus foliaceus and the presence of antibodies against desmoglein 1 was recently reported.28
In addition to idiopathic p. foliaceus, drug-induced variants, notably due to penicillamine, may also be encountered (Fig. 5.24). A localized form may also be associated with topical drugs such as imiquimod and has been reported following radiation therapy.29–32 Pemphigus foliaceus is rarely associated with an underlying malignancy including non-Hodgkin’s lymphoma and esophageal cancer.33,34
Pathogenesis and histological features
Similar to other variants of pemphigus, p. foliaceus is an immunologically mediated disease. Examination of perilesional skin by direct immunofluorescent techniques reveals in vivo-bound immunoglobulin (usually IgG) and often complement (C3) in the intercellular region of the epidermis.1 Abundant antigen in the follicular outer root sheath and germinal matrix may account for the marked scalp involvement typical of pemphigus.35
Indirect immunofluorescent techniques show that the sera of patients with p. foliaceus contain an IgG antibody that reacts with the intercellular region of normal squamous epithelium.36 IgG4 predominates followed by IgG1.37,38 IgG3 is also sometimes present. This may be of importance since IgG3 is the most efficient activator of complement.37 Some 60–70% of patients have positive indirect immunofluorescence.39
The p. foliaceus antibody binds to a 160-kD desmosomal cadherin, designated desmoglein 1 (Dsg1).40,41 The sera of p. foliaceus patients bind to the extracellular amino terminal domain of bovine Dsg1 whereas sera from both p. vulgaris and p. vegetans patients react with the intracellular domain of Dsg1.42,43 Compared with p. vulgaris, immunofluorescence studies on the sera of p. foliaceus tend to show more staining in the superficial epidermis, correlating with the level of the split.44,45 Conversely, the sera from patients with p. vulgaris show more affinity for the lower epidermis. Anti-Dsg1 antibody is pathogenic.46 Injection of purified anti-Dsg1 antibodies from sera of patients with p. foliaceus into neonatal mice induces subcorneal acantholysis in a pattern typical of p. foliaceus.47 Acantholysis is thought to be the result of an antibody-mediated cellular response rather than purely the result of steric hindrance.48 Internalization of nonclustered Dsg1 has been put forward as a possible mechanism resulting in lack of newly formed desmosomes rather than a disruption of pre-existing structures.49 Increasing evidence suggests that the blistering is the result of the activation of p38 mitogen-activated protein kinase-dependent signaling by the p. foliaceus IgG antibodies.50 Rarely, patient sera contain additional IgG antibodies directed against desmoglein 3 (Dsg3) and the presence of additional IgA antibodies against Dsg1 as well as Dsg3 has also been detected.3,51,52 Furthermore, three patients have been reported with clinical and histological features of p. foliaceus but direct immunofluorescence findings reminiscent of p. erythematosus. Antibodies recognizing bullous pemphigoid antigen1 (BP230) as well as a 190-kD protein co-migrating with periplakin were detected in these patients in addition to anti-Dsg1 antibodies.53 The use of D-penicillamine may be associated with the acquisition of a pemphigus-like antibody and the development of p. foliaceus.54
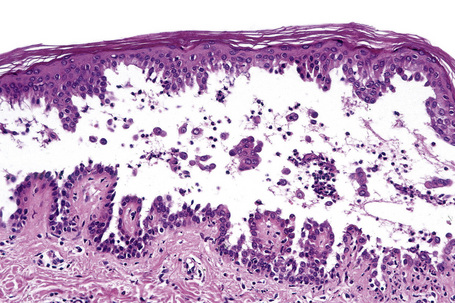
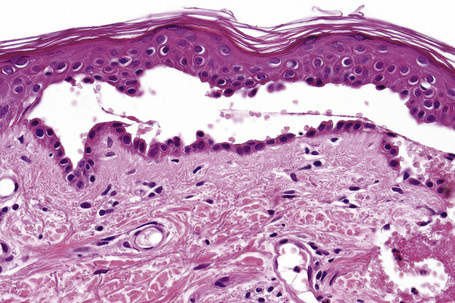
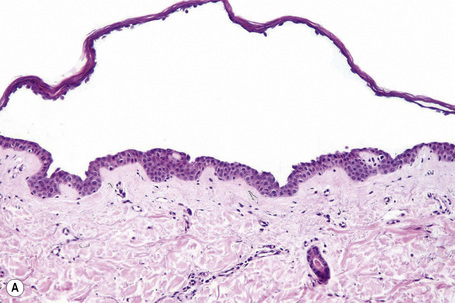
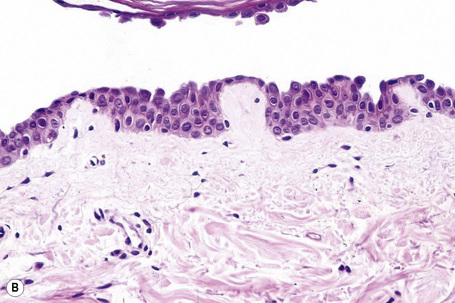
Since the blisters of p. foliaceus are superficial, they are therefore fragile and it is often very difficult to obtain an intact lesion for diagnosis. Patients commonly have erosions without blisters, and frequently the clinician does not suspect a bullous disorder. Usually, the cleft or blister lies within the granular layer or beneath the stratum corneum (Fig. 5.25). The roof of the fragile blister is often not present, having sloughed either before or after biopsy. Acantholysis is frequently difficult to detect, but usually a few acantholytic cells can be found attached to the roof or floor of the blister. In those cases where the blister is missing, a careful inspection of the hair follicles may reveal focal acantholysis. Sometimes the blister contains numerous acute inflammatory cells (Fig. 5.26), particularly neutrophils, which can make distinction from subcorneal pustular disorders, including bullous impetigo, a dermatophyte infection, candidiasis, pustular psoriasis, and subcorneal pustular dermatosis especially difficult.55,56 Eosinophilic spongiosis may also be seen.57
Differential diagnosis
The histological features in the superficial forms of pemphigus may be easily overlooked and, since bullae are often not appreciated by the clinician, the unwary pathologist may not consider a bullous disorder when evaluating the biopsy. A high index of suspicion is therefore critical. The differential diagnosis of superficial pemphigus includes bullous impetigo, staphylococcal scalded skin syndrome, IgA pemphigus, and subcorneal pustular dermatosis (Table 5.3). Distinction depends upon a careful consideration of the clinical information, the results of bacterial culture, and immunofluorescent studies.
Table 5.3 Differential diagnosis of superficial pemphigus: conditions characterized by subcorneal pustules
| Superficial pemphigus |
| IgA pemphigus |
| Subcorneal pustular dermatosis |
| Pustular psoriasis |
| Reiter’s syndrome |
| Pustular drug reaction |
| Bullous impetigo |
| Staphylococcal scalded skin syndrome |
| Pustular fungal infection |
Endemic pemphigus foliaceus (fogo selvagem)
Clinical features
Fogo selvagem (Brazilian pemphigus foliaceus, ‘wild fire’, endemic pemphigus foliaceus) is endemic in regions of Brazil and has also been documented in other areas of Central and South America including Colombia, El Salvador, Paraguay, Venezuela, and Peru.1–11 An endemic area has also been described in Tunisia.12,13 The condition is associated with poverty and malnutrition and particularly affects children and young adults. Results from a more recent epidemiological study demonstrated disease manifestation also in patients of higher socioeconomic class and urban areas.14 There is a striking familial incidence.4 Most cases are found along major rivers, and people especially at risk include farmers and workers involved in land clearing and road construction.2 It appears that the majority of patients live at an altitude of between 500 and 800 meters, and that their homes are generally within 10–15 kilometers of running fresh water and in the path of prevailing winds, thus suggesting a likely insect vector.4,15 In support of this, a case-controlled epidemiological study has provided evidence that bites by the black fly (family Simuliidae) are a significant risk factor for development of the disease and it has been proposed that a component of the saliva may trigger an antibody response in susceptible individuals.16–18 Simulium nigrimanum, which is found in the same areas in which Brazilian fogo selvagem occurs, has been identified as being the likely species involved.17
The clinical presentation of fogo selvagem has been divided into a number of categories including localized and generalized forms:2,4
With resolution, patients may sometimes develop hyperpigmentation.19 The antibody does not cross the placenta and therefore neonatal disease is not a feature.20 Patients with fogo selvagem appear to have no increased risk for other concomitant autoimmune disorders.21
In contrast to Brazilian fogo selvagem, endemic disease in the area of El Bagre, Colombia, shows several unusual and distinguishing features.22 The disease affects an older population with a strong male predilection and clinical features reminiscent of pemphigus erythematosus. In addition to the more classical presentation, patients develop hyperkeratotic plaques on the face, chest, and back reminiscent of discoid lupus erythematosus as well as an erythematous macular lesion in a butterfly-like distribution in the central face.22 Active disease is also accompanied by conjunctivitis. The disease also shows characteristic immunological and histological changes, which are discussed below.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree