CHAPTER 30 Abnormalities in immunoglobulin synthesizing cells
Multiple myeloma
Epidemiology and etiology
MM represents 10–15% of all hematologic malignancies and 1% of all cancers, with an incidence of 2/100 000.1 The incidence increases with age, with approximately 40% of patients presenting under the age of 60 years and only 2% of cases occurring before the age of 40 years. There is a moderate excess in males. Geographic and racial differences play an important role, as the disease is more common in black people than Caucasians and has a low incidence in Chinese people. These rates are retained after migration to new countries, suggesting an inherited rather than an environmental explanation for the differences. Epidemiological studies have been carried out to identify environmental risk factors.2,3 An association with radiation exposure is seen in survivors of the World War II atomic bombs, as well as in occupationally and therapeutically exposed groups. There is also a suggestion of an association with farming, paper production, woodwork and exposure to a variety of chemicals including petroleum, benzene and materials associated with plastic and rubber manufacture. In addition to traditional epidemiology studies, there is much interest in determining whether inherited polymorphic variation can influence the development of MM or a patient’s response to treatment.2,3 To date most studies have been small and have concentrated on single nucleotide polymorphisms (SNPs) that affect the function of genes already known to be important in MM pathogenesis (e.g. immune response and cytokine genes). The introduction of newer high-throughput technologies with near complete genome coverage will enable this important area to be investigated further over the next few years.
Biology
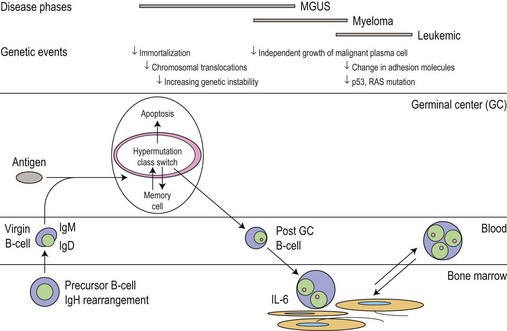
The cell of origin
The main phenotypic features of myeloma PC include abnormal localization within the BM, replacement of normal BM elements, and dysregulation of Ig secretion. Normal PC in BM are derived from cells that have passed through a germinal center in a lymph node or other organ. Within the germinal center, cells undergo somatic hypermutation, class switching of the Ig gene, and selection by antigen-binding affinity; only cells with high binding affinity survive to become PC. In myeloma the Ig genes from individual plasma cells show the same pattern of somatic hypermutation, consistent with the clonal expansion of a single postgerminal center B-cell.4,5 The high incidence of translocations involving the switch region on chromosome 14 would also indicate that the final molecular oncogenic event occurs late in B-cell development. This contrasts with monoclonal gammopathy of unknown significance (MGUS) where there is intraclonal variation in the pattern of mutation, suggesting transformation of a virgin or memory B-cell with progeny which continue to pass through the normal process of germinal center selection before becoming plasma cells (Fig. 30.1).
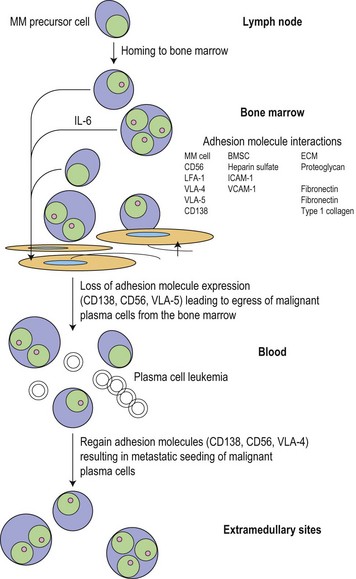
Biology and growth signaling
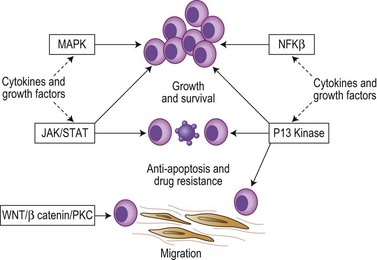
Following the transformation of the proliferative ‘plasmablastic’ cell located in the germinal centre, adhesion molecules mediate homing of the immortalized progeny of this cell from the lymph node to specialized niches within the BM, where maturation into a malignant PC occurs (Fig. 30.2). Further genetic hits lead to loss of tumor suppressor genes, expression of oncogenes and alteration in cell cycle control, resulting in a proliferative advantage for the MM cell and disease progression. Binding of myeloma cells to the BM stroma occurs and localizes tumor cells within the BM microenvironment. This binding to stroma results in an increase in the paracrine transcription and secretion of cytokines (particularly interleukin (IL)-6, insulin-like growth factor 1 (IGF1) and vascular endothelial growth factor (VEGF)), mediating myeloma cell growth and survival, and protection from drug-induced apoptosis (Fig. 30.3).6,7
The cytokines IL6, IGF1 and VEGF together with direct myeloma cell to cell contact trigger signaling via the Ras/MEK/MAPK pathway resulting in myeloma cell growth, survival and drug resistance.7–9 Mutations affecting these pathways result in cytokine independent myeloma cell growth, the development of drug resistance and extramedullary disease. IL6 and IGF1 also signal via the PI3kinase-AKT-mTOR pathway, mediating myeloma growth, cell cycle and apoptosis.7–9 Activation of mTOR results in phosphorylation of P70S6 and 4E-BP1, which plays a key role in regulating the translation of cyclin D and c-myc, two proteins known to be central to myeloma pathogenesis. IL6 also triggers signaling via the JAK/STAT3 pathway and triggers drug resistance via activation of RAFTK and the mitochondrial release of Smac.
Nuclear factor kappa B (NFκB) signaling is important in B-cell biology and most myeloma cell lines demonstrate activation of NFκB leading to increased myeloma cell growth and survival. The pathway is also the target of multiple mutational events with 20% of cases harboring mutations or deletions of key inhibitory members of both the canonical and non-canonical pathways.10,11 A further key pathway is the TNFα superfamily (SDF1, CD40, BAFF, APRIL). Although the direct effect of TNFα on cell proliferation is modest, it markedly up-regulates the secretion of IL6 from BM stromal cells leading to dramatic increases in myeloma cell growth. TNFα also induces NFκB dependent expression of adhesion molecules increasing binding between myeloma cells and stromal cells resulting in protection from drug-induced apoptosis. In addition, CD40 mediates a p53 dependent increase in myeloma cell growth and PI3kinase/AKT/NFκB dependent migration.
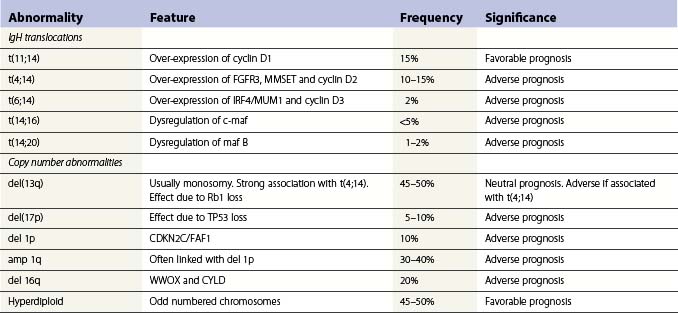
Cytogenetic and molecular abnormalities
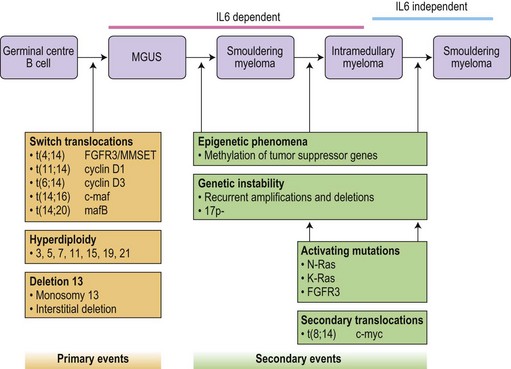
Translocations occurring as a result of aberrant class switch recombination events are the earliest known genetic events in MM (Fig. 30.4).12 The molecular characterization of the common recurrent translocations, t(4;14), t(11;14), t(14;16) and t(6;14), has identified a number of deregulated oncogenes including FGFR3/MMSET, cyclin D1, c-MAF and cyclin D3, respectively. In addition to switch translocations, secondary events such as chromosomal copy number alterations are common and genetic instability occurs resulting in deletions (e.g. 13q-, 17p-/p53, 1p-/CDKN2C, 16q-/CYLD/WWOX, NFκB inactivation/BIRC/TRAF3), activating mutations (e.g. NRas, FGFR3) and secondary translocations (e.g. t(8;14)). This has led to a molecular classification of myeloma based on the presence of switch translocations, hyperdiploidy and deregulation of the D group cyclins (Table 30.1).13 Two broad groups of patients can be recognized: a hyperdiploid group where there is a low incidence of switch translocations (<30%) and a non-hyperdiploid group where the incidence is high (>85%). Chromosome 1 abnormalities, usually 1q gain and 1p loss, are among the most prevalent cytogenetic abnormalities. The majority involve rearrangements located in the pericentromeric regions of the chromosomes and form jumping translocations. The actual gene responsible for the biological effects is uncertain although CSK1B and CDKN2C have been suggested as candidates. Recent studies have also suggested epigenetic changes contribute to the disease phenotype with patients showing overexpression of MMSET, a protein with histone methyl transferase activity and mutations in UTX, a histone demethylase.15
Advances in technology have now enabled the correlation of these genetic features with clinical outcome and has identified a series of distinct clinical subgroups. One such group are patients with the t(4;14)(p16.3;q32), which is present in 10–15% of myeloma cases.16,17 The translocation leads to dysregulation of two potential oncogenes, fibroblast growth factor receptor 3 (FGFR3) and multiple myeloma SET domain (MMSET). Myeloma carrying t(4;14) has a distinct gene expression profile and clinical profile with a short duration of response to chemotherapy, resistance to conventional alkylating agents and poor overall prognosis compared with other translocation groups. Patients with a t(11;14), present in 15% of cases, also have a distinct clinical phenotype. This translocation results in the up-regulation of cyclin D1, is associated with lymphoplasmacytic morphology, CD20 expression, λ light chain usage and low CD56.18 In addition, rare IgM myeloma often carry t(11;14).19 The prognostic significance depends on the series examined but ranges from neutral to favorable.
Patients with deletion of 17p also have a distinct clinical phenotype with a high incidence of extramedullary disease and aggressive course, short remissions and a short overall survival.20,21 Abnormalities of both the long and short arm of chromosome 1 have been linked with short survival, and gene expression profiles identifying patients with high risk disease are highly enriched for genes located on this chromosome. The prognostic significance of chromosome 13 deletion is more controversial with some studies showing a strong prognostic significance whereas other studies demonstrate little effect. This appears to depend on the detection method used to determine the presence of the abnormality, chromosome banding versus interphase FISH. In addition, all patients with a t(4;14) demonstrate deletion of chromosome 13, and as t(4;14) patients tend to have a poor prognosis this ‘linked effect’ may account for some of the differences.
Bone disease
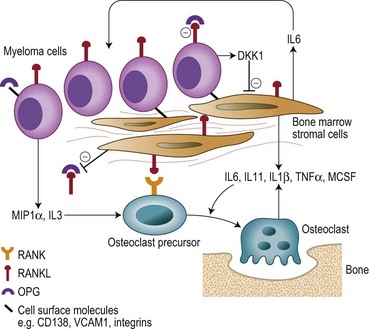
Bone destruction in MM is a prominent feature and causes considerable morbidity. Bone remodeling is a continuous process of resorption by osteoclasts and the subsequent formation of new bone by osteoblasts. In myeloma there is an increase in the number of osteoclasts and bone resorption in areas of the marrow adjacent to abnormal PC, but not in those areas adjacent to normal BM cells. New bone formation is also reduced when the tumor burden in the BM is high, and the combination of increased resorption and decreased formation leads to an uncoupling of normal bone remodeling.22,23 The central players involved in this process include: the receptor activator of NFκB (RANK); RANKL, the ligand for RANK; and osteoprotegerin (OPG) (Fig. 30.5). RANKL exists in a membrane and soluble form and via its receptor RANK increases bone resoprtion by increasing osteoclast formation and activity. OPG prevents bone resorption by acting as a decoy receptor preventing the binding of RANKL to RANK thereby inhibiting the up-regulation proliferation and fusion of osteoclast precursors to produce mature osteoclasts. A number of other cytokines and chemokines modify the BM microenvironment leading to an upregulation of RANKL by both stroma and osteoblasts including IL6, IL1β, IL11, lymphotoxin, Tumor necrosis factor (TNF)-α, and macrophage inflammatory protein 1α (MIP-1α), further perpetuating the cycle of bone destruction. In addition the increased osteoclast activity results in the secretion of tumor growth factor (TGF)-β, IL-6, β-fibroblast growth factor (FGF) and IGF-1 from the BM matrix in turn leading to further myeloma cell growth.
Diagnostic criteria
An international classification system has recently replaced a number of different diagnostic criteria to aid in the classification of the monoclonal gammopathies.24 Due to overlapping features, myeloma must be distinguished from the other disorders characterized by the presence of a monoclonal protein including MGUS, Waldenström’s macroglobulinemia, non-Hodgkin lymphoma, light-chain amyloid, idiopathic cold agglutinin disease, essential cryoglobulinemia, and heavy-chain disease. Some of these disorders are discussed later in this chapter, while non-Hodgkin lymphomas are discussed in Chapter 29. The majority of MM patients will have an M protein in the serum >30 g/l and/or BM clonal plasma cells >10% (Table 30.2). Patients are then classified depending on the presence or absence of end organ damage related to the plasma cell proliferative process (Table 30.3). Symptomatic patients have evidence of related organ or tissue impairment (end organ damage) (ROTI). Examples include raised calcium levels, renal insufficiency, anemia and bone lesions. Generally these patients require urgent therapy. Asymptomatic patients have no evidence of ROTI and usually undergo close monitoring with treatment initiated at disease progression. The term asymptomatic myeloma tends to include patients previously classified as having smoldering myeloma or Durie–Salmon stage I disease.
Table 30.2 Monoclonal (M) protein incidence and type24
| Type | Incidence | |
|---|---|---|
| Serum M protein | Detectable in over 90% of patients using immunofixation | |
| Urinary M protein | Present in over 75% of patients | |
| M protein type | IgG | >50% |
| IgA | 20% | |
| IgD | 2% | |
| IgE | 1% | |
| Light chain only | 20% | |
| Non-secretory disease | 3% | |
Table 30.3 Myeloma-related organ or tissue impairment (end organ damage)24
| *Calcium | >0.25 mmol/l above the upper limit of normal or >2.75 mmol/l |
| *Renal insufficiency | Creatinine >173 mmol/l |
| *Anemia | Hemoglobin 2 g/dl below the lower limit of normal or hemoglobin <10 g/dl |
| *Bone lesions | Lytic lesions or osteoporosis with compression fractures |
| Other | Symptomatic hyperviscosity, amyloidosis recurrent bacterial infections (>2 episodes in 12 months) |
* CRAB, calcium, renal insufficiency, anemia or bone lesions.
Clinical features
Bone disease
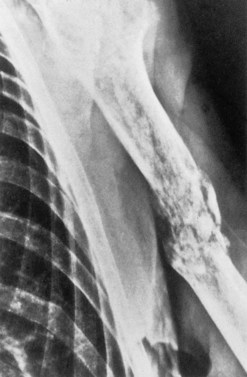
The accumulation of myeloma cells within the cavity of bones in the axial skeleton produces bone pain and destruction. The pain arises in the axial skeleton, and loss of height due to collapse of vertebrae and kyphosis are common. Although bone pain may be gradual in onset, pathologic fractures are frequent and usually indicated by the sudden onset of local tenderness and pain. Seventy per cent of patients will have evidence of bone disease at presentation and in almost all cases the bone lesions are osteolytic (Fig. 30.6), but a minority of patients (2%) have osteosclerotic lesions. The majority of patients also have diffuse osteopenia. Bone resorption leads to increased calcium in 20–40% of patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree