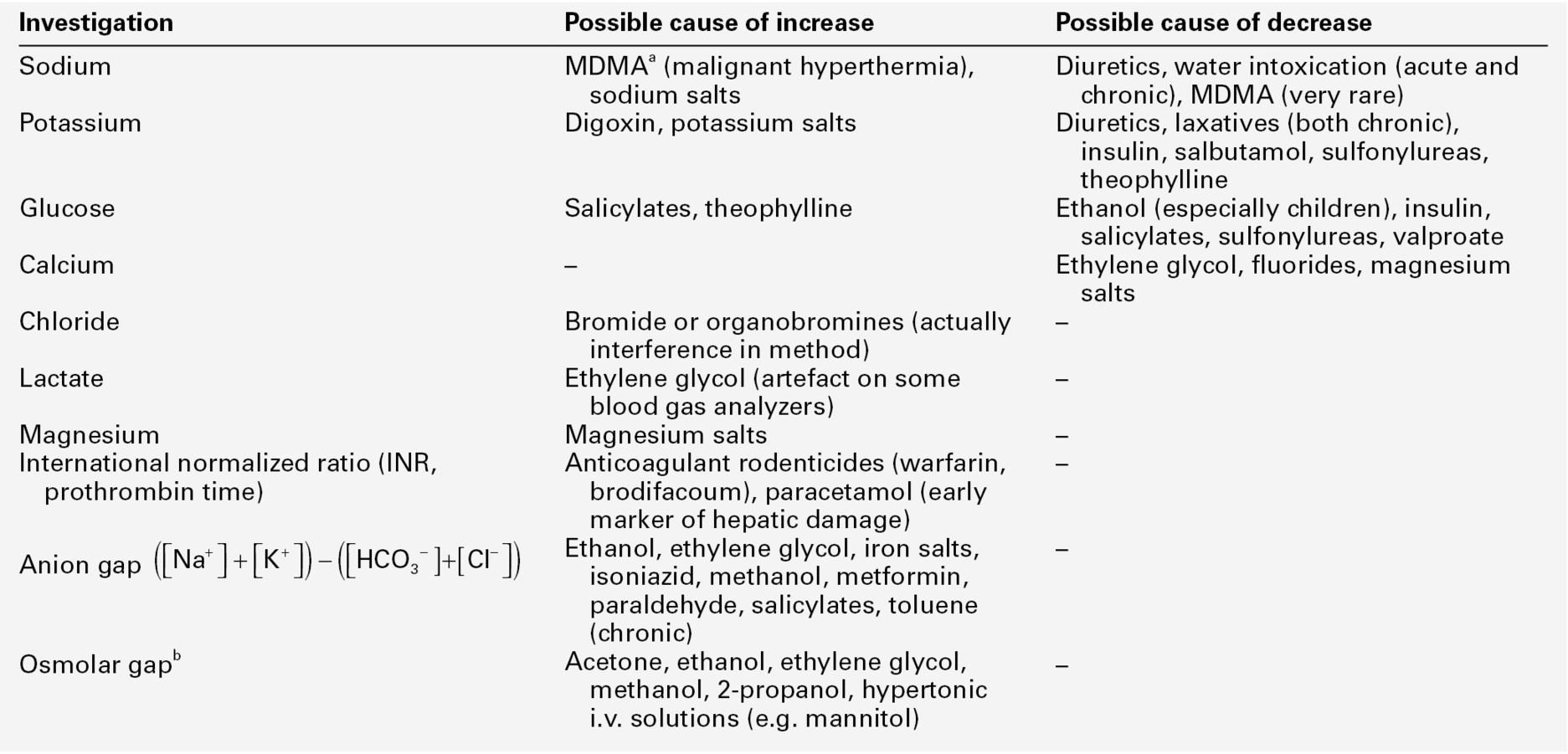
CHAPTER 44 Robert J. Flanagan; Sarah Belsey; Terhi Launiainen CHAPTER OUTLINE Forensic biochemistry can be defined as the application of biochemical assays in the service of the courts. Examples include DNA analysis for human identification and methods for the detection of trace evidence, such as the Kastle–Mayer (phenolphthalein/hydrogen peroxide) and luminol reactions used to detect the presence of blood. This chapter, however, focuses on laboratory measurements rather than methods. Many of these are standard laboratory procedures, while others are specific to forensic work. The range of forensic situations that the clinical biochemistry laboratory may be asked to help with is wide and, as in all such work, the results of tests can usually be properly interpreted only when considered together with all the available evidence. Post-mortem biochemistry has a role in investigating the cause of death in some apparently natural deaths, including both diabetic and alcoholic ketoacidosis, deaths that may have involved a prolonged stress response such as hypothermia, as well as in the diagnosis of disease processes such as early myocardial infarction, which may be difficult to diagnose by physical examination. There is clearly considerable overlap between clinical and forensic toxicology, in that some endogenous substances can be used as poisons (e.g. sodium chloride, potassium chloride and insulin) and in some instances, suspicion of poisoning may be aroused by abnormal biochemical results (see Table 44.1). TABLE 44.1 Some laboratory investigations commonly requested on blood samples that may arouse suspicion of poisoning a Methylenedioxymetamfetamine. Forensic biochemistry has an important role in the investigation of deaths and serious injuries occurring in hospital. Assault committed within hospital may involve poisoning, and can range from murder (intent to kill), through manslaughter (culpable homicide) and attempted murder, to malicious poisoning (usually of a child or an elderly relative). Iatrogenic poisoning may range from relatively minor drug administration errors, to catastrophes such as asphyxia caused by an anaesthetic error. The results of an analysis, or residual or unused samples, or even apparatus used in giving the drug, may be required by officers acting on behalf of the courts. Therefore, in all cases, careful specimen labelling and handling, reporting and laboratory record-keeping is important. The standard of completion of request forms and sample labelling is still very poor in some hospitals. This can cause many problems if samples are required by the police or coroner. Knowledge of the limitations of the analytical methods used is also important – an enzymatic ethanol assay is not as selective as headspace gas chromatography, for example. One practical problem is that samples may be collected, assays performed and results reported before the need for forensic investigation has become apparent. Another problem is that all that may be available are specimens collected after death (see Box 44.1), although in general it is information on an analyte’s concentration prior to, or at the time of death that is required. In this case, the likelihood of agonal or post-mortem change, and indeed, sample contamination during collection, must be taken into account when interpreting results. An associated problem is that there are often no reference ranges for fluids such as vitreous humour, pericardial fluid, or synovial fluid since such samples are, for practical purposes, rarely available during life, except from laboratory animals. Method validation is also compromised by this same lack of reference material. Furthermore, the time needed for analyte equilibration between plasma and, for example, vitreous humour during life remains unknown. Of course the laboratory, too, can be the subject of forensic investigation if laboratory error of whatever nature comes under scrutiny of the courts (see Box 44.2). Examples here include the use of inappropriate analyses, delayed analyses, sample mix-ups and errors in reporting, including the use of inappropriate units. In all cases, it is best to write out units in full, for example ‘milligrams per litre’, rather than using symbols, when producing reports for the courts. The laboratory should provide clear guidance as to the significance of a result, especially when different units may be used. Patients have died when a paracetamol result reported in mg/L has been assumed to be in mmol/L. Mass units (SI) should be used for drugs except for lithium, thyroxine and methotrexate, where molar units should be employed. For metals/trace elements and for alcohol (ethanol), either molar or mass units may be employed. However, for forensic purposes, including the regulations governing occupational lead exposure, mass units are often the rule and the laboratory should remember this when providing interpretation of results. For clinical purposes, ethanol is often reported as mass units per litre (mg/L), but for forensic purposes, at least in the UK, ethanol is still reported as mg/100 mL (mg%). Information recorded on the sample container at the time the sample is collected should include the names (first and family or last name) and date of birth, patient or post-mortem number and the date and time of collection. This information, together with details of the collection site in post-mortem work, and the sample type (including a note of any preservative), and any other appropriate information, should be recorded on an accompanying assay request form (see Box 44.3). The date and time of receipt of all specimens by the laboratory should be recorded and a unique identifying number assigned in each case. Any residual specimen should be kept securely at − 20 °C or below until investigation of the incident has been concluded. In forensic work, it is important to be able to guarantee the identity and integrity of the specimen from when it was collected through to the reporting of the results, although this ideal is often not attained in normal clinical laboratory practice. ‘Chain of custody’ is a term used to refer to the process used to maintain and document the history of the specimen (see Box 44.4). Procedures for appropriate storage of samples that may be required for forensic analysis and for authorizing and documenting the release of such samples on request of a coroner, for example, must also be in place. Ideally, samples should be protected during transport by the use of tamper-evident seals and should be submitted in person to the laboratory by the coroner’s officer or other investigating personnel. If storage is to be at − 5 to − 70 °C, basic precautions to preserve sample integrity, including labelling, must be undertaken (glass tubes will break if over-full when frozen). The requirements of the UK Human Tissue Act, or other relevant legislation on the retention and storage of pathological samples, must be met. Many analytes of toxicological interest (and their metabolites) also occur naturally in the body and hence, reference ranges, ‘cut-offs’ or other means of delineating an exogenous source for the compound(s) of interest must be adopted. Examples include acetone, carbon monoxide (measured as % carboxyhaemoglobin) ethanol, γ-hydroxybutyrate (GHB), insulin, iron, potassium, sodium and testosterone. Testosterone continues to be abused by athletes and this remains the most common adverse finding declared by World Anti-Doping Agency accredited laboratories; the practice is usually detected by the demonstration of an elevated testosterone:epitestosterone ratio and by procedures to ensure that such a finding is not ‘natural’ for a particular athlete. Numerous other endogenous agents have been used in an attempt to enhance performance or to mask use of other agents in sport but further discussion of this topic is beyond the scope of this chapter. γ-Hydroxybutyrate (GHB), and its precursors γ-butyrolactone (GBL) and 1,4-butanediol, are used to improve athletic performance, as intoxicants and sometimes in assault such as drug-facilitated sexual assault (which includes rape). As with alcohol, voluntary ingestion always has to be considered (Box 44.5). γ-Hydroxybutyrate exposure may also arise from unexpected sources (Box 44.6). The substance is practically odourless and tasteless and has a short plasma half-life (20 min or so), making covert administration hard to detect. Quantitation is needed as GHB is an endogenous compound. A urine cut-off of 10 mg/L is recommended in attempting to differentiate endogenous GHB excretion from deliberate GHB/GBL administration. Unusually, the urine cut-off seems more reliable than a measure of plasma GHB, but by ~ 12 h after GHB ingestion, the concentration in urine is usually < 10 mg/L. For post-mortem blood, the cut-off is generally taken as 50 mg/L because of the likelihood of post-mortem GHB production.
Forensic biochemistry
INTRODUCTION
SAMPLES AND SAMPLING
POISONING WITH ENDOGENOUS AGENTS
γ-Hydroxybutyrate
Basicmedical Key
Fastest Basicmedical Insight Engine



