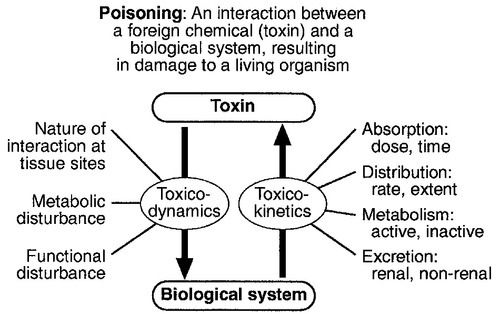
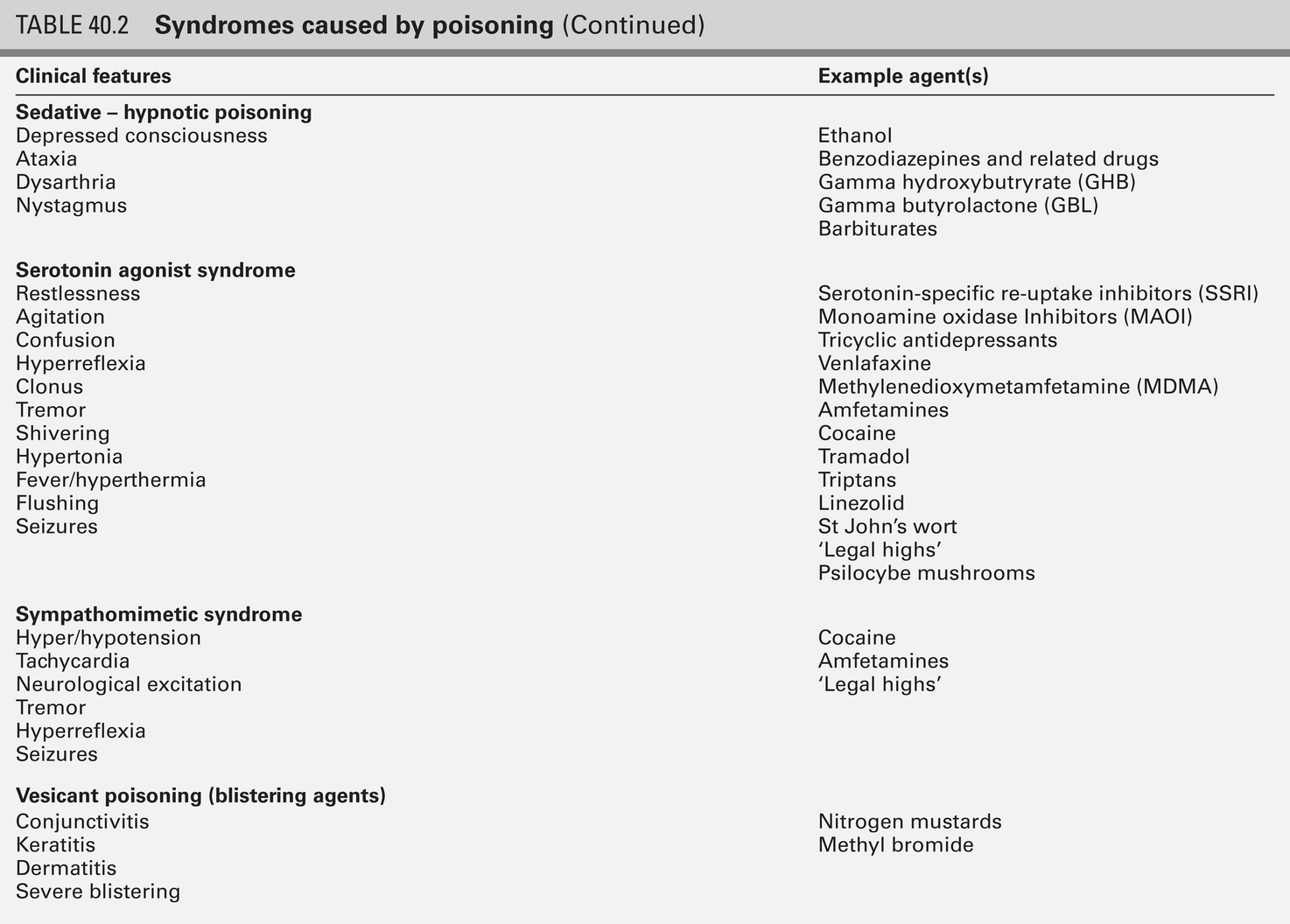
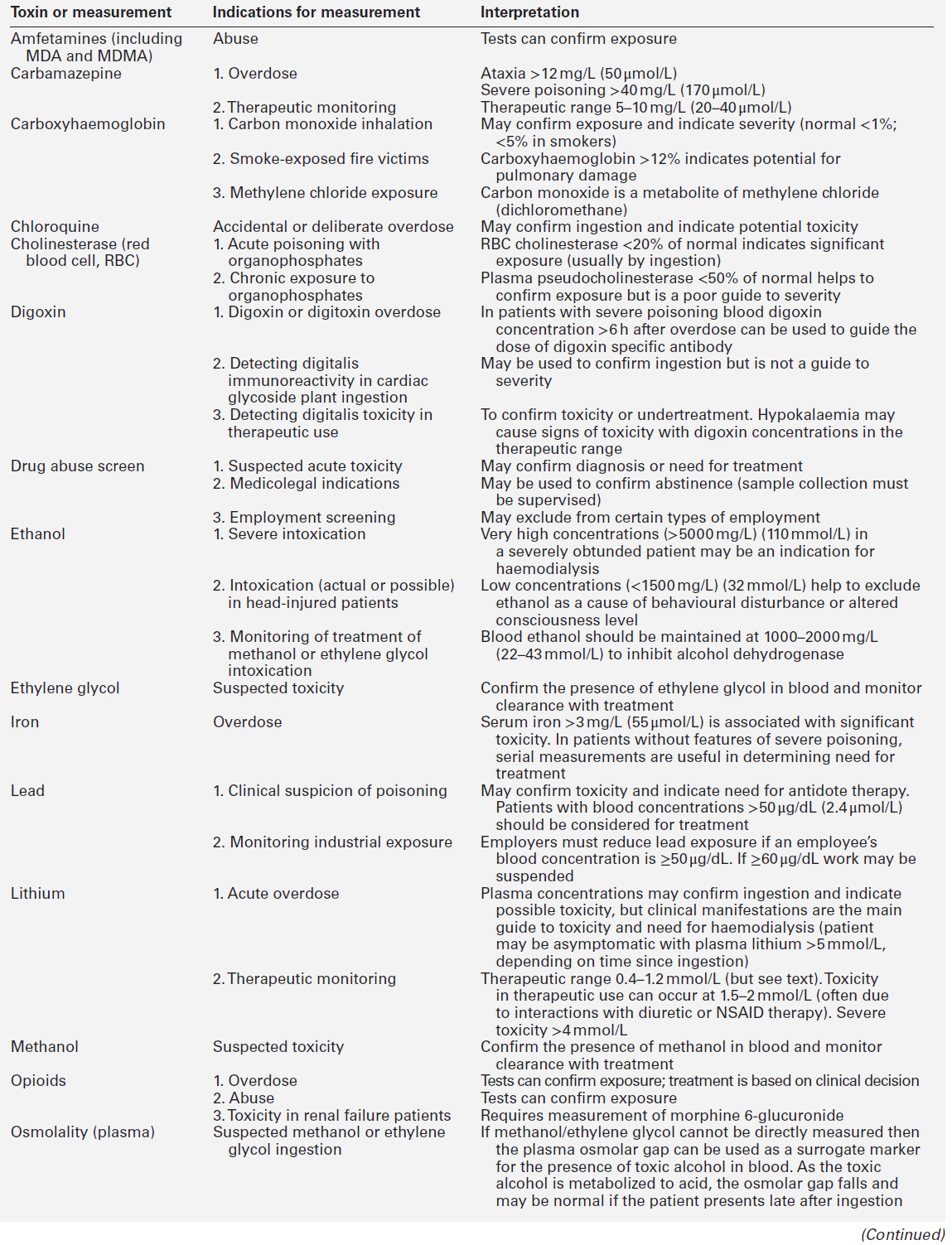
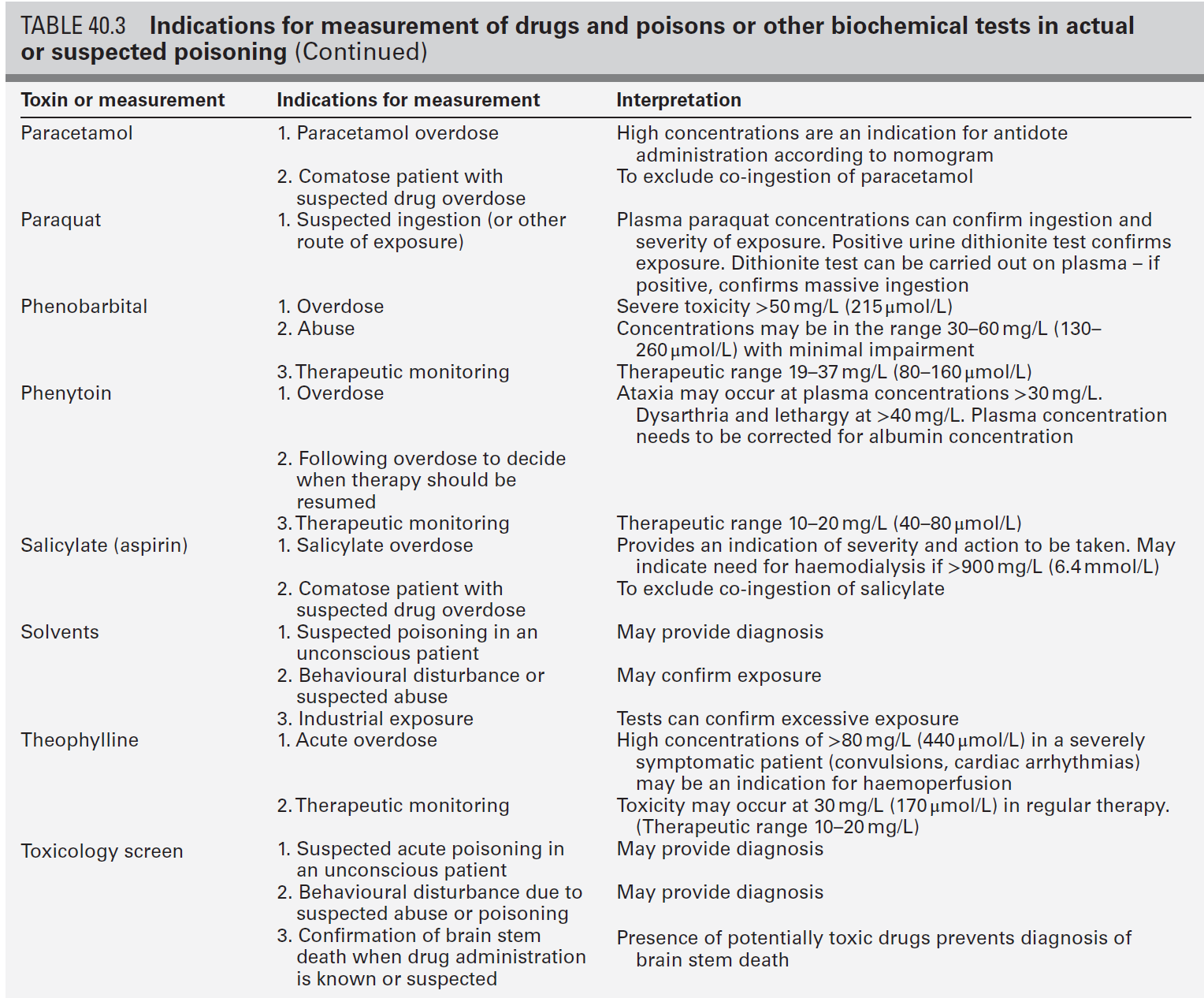
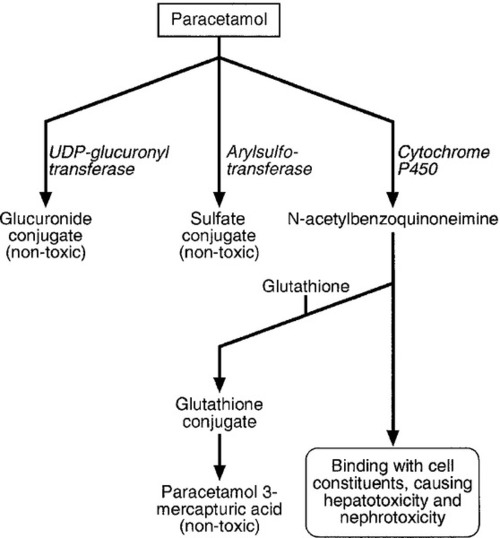
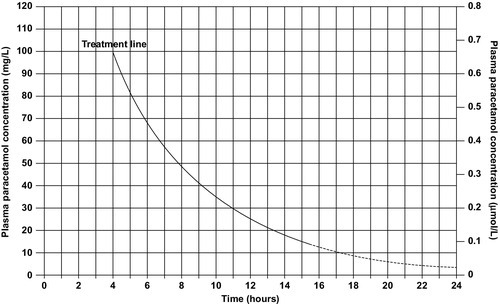
CHAPTER 40 CHAPTER OUTLINE Poisoning can be defined as an interaction between a foreign chemical (toxin) and a biological system that results in damage to a living organism. In general, the medical profession has been more concerned with the acute effects of toxins and the clinical management of toxicity, but chronic effects of toxins have much more importance on a global scale. For example, acute ingestion of ethanol may result in intoxication, which can cause death directly from its acute depressant effects and also through intoxication-related accidents and violence. However, alcohol-related liver disease, myopathy, hypertriglyceridaemia and cancers are all effects of chronic alcohol abuse and together, cause far more deaths than the acute effects of alcohol. Other types of chronic or delayed effects of toxins include mutagenicity, carcinogenicity and teratogenicity. For example, many cases of bronchogenic carcinoma are a direct result of exposure to cigarette smoke. Increasing concerns about environmental contamination by chemicals has added a further dimension to toxicology, especially as many chemicals, such as pesticide residues, may be found in small amounts in the general population, who do not appear to be suffering any ill effects. The interaction between a toxin and the biological system is highly complex. Once the biological system has been exposed to the toxin, the toxin is subject to various processes. In the case of a drug, this is usually termed pharmacokinetics, but in the case of a poison, the term toxicokinetics may be used. This includes absorption, for which the route of administration is of great importance, as are the dose of the toxin and the duration of exposure. Once a toxin has entered the bloodstream, its distribution depends to a large extent, on its physicochemical characteristics, including its degree of lipid or water solubility and its degree of ionization. Metabolism is a further important process, which usually renders toxins inactive, but which may also convert some substances of low toxicity into highly toxic metabolites. Classic examples of this include paracetamol (acetaminophen), methanol and ethylene glycol. Finally, toxins are excreted, although some remain within the body for many years. All of these processes determine the amount of toxin that interacts with tissues to produce a toxic effect. The study of the interaction between toxins and tissues is called toxicodynamics. An understanding of the nature of this interaction is essential for an intelligent approach to the diagnosis and management of poisoning. This interaction may cause functional and metabolic disturbances that produce the characteristic clinical features of poisoning and the laboratory changes that may be essential for diagnosis or management. These changes are summarized in Figure 40.1. This chapter covers several of the more common types of acute poisoning and also some aspects of chronic poisoning. Clinical features are given in some detail, as these frequently integrate with the metabolic and biochemical changes. A number of drugs, such as tretinoin, should not be taken by women intending to become pregnant because of their known teratogenicity. Other drugs, such as phenytoin and several other anticonvulsants, are teratogenic but their continued use may be considered necessary during pregnancy. As far as poisoning and overdose during pregnancy are concerned, the fetus generally only suffers damage secondary to the mother’s illness, and the management is no different from that of overdose in non-pregnant women. One exception is carbon monoxide, which has a higher affinity for fetal haemoglobin than adult haemoglobin; the mother should be treated aggressively even in apparently mild poisoning. Antidotes such as acetylcysteine or desferrioxamine should not be withheld because the patient is pregnant. During the neonatal period, particularly in premature infants, there is a risk of iatrogenic overdose because of the poor metabolic and excretory capacity relative to body weight. Particular care should be taken with drugs such as theophylline, digoxin, chloramphenicol and morphine. The commonest cause of poisoning between the ages of one and four is termed accidental poisoning because of the natural exploratory activities of children at this stage of life. Each year, this results in 20 000 admissions to hospital in Britain, but only about ten fatalities. The commonest agents involved are paracetamol and oral contraceptives, but most fatalities occur from tricyclic antidepressants, salicylates, iron, methadone and quinine. Another form of poisoning in this age group is non-accidental injury, which is usually inflicted by a carer such as the mother, and the child may present with repeated episodes of floppiness and collapse, depending on the substance responsible. This is most commonly with a drug prescribed for the mother, though salt and other readily available chemicals have been used (see Chapter 44). Later in childhood, substance abuse, particularly volatile substance abuse (‘glue sniffing’), can cause serious problems. There are about 30 deaths each year in Britain from this cause. Acute and chronic alcohol toxicity are often underestimated in this age group. Intentional drug overdose is common, but suicide is rare. In early adult life, particularly in females, drug overdose in the form of parasuicidal gestures is common, though rarely fatal. In later adult life, suicidal intent is commoner and parasuicidal gestures are less frequent. The commonest causes of suicidal poisoning in England are tricyclic antidepressants, analgesic drugs and carbon monoxide. Male suicides are approximately three times as frequent as female suicides. Accidental poisoning in adults is usually domestic, the commonest agent being carbon monoxide, or industrial, in which a wide range of chemicals may be involved. Homicidal poisoning, though rare, may go undetected if it is not suspected. Many types of poisoning involve a highly specific interaction between the toxin and one type of tissue. This has led to a ‘target organ’ oriented approach in toxicology, which is of great help in understanding poisoning, where damage to a single organ or tissue produces a characteristic clinical and pathological picture. Examples include poisoning with paracetamol, salicylates, cholinesterase inhibitors and cardiac glycosides. In other instances, the type of toxicity may be less clearly definable. The acute effects of ethanol are mainly due to its central nervous depressant effect. Cocaine, tricyclic antidepressants, many volatile substances, some β-adrenergic blocking drugs and dextropropoxyphene can all produce cardiotoxicity due to a quinidine-like sodium channel blocking effect. However, the clinical features are definable as a toxic effect on the heart. An important way in which many poisons can affect the organism is by interference with the oxygen pathway from the inspired air to cellular respiration. Thus, a reduction in the oxygen content of the inspired air may cause acute hypoxaemia, rapidly leading to collapse and coma. Interference with the mechanics of respiration by poisons may result in hypoxaemia and hypercapnia (type II respiratory failure), while disturbances of oxygen transfer produce hypoxaemia alone (type I respiratory failure). Even if the oxygen content of the air is not diminished and respiration is functioning adequately, disturbances to other processes may prevent oxygen reaching its site of action (see Chapter 5). The oxygen-carrying capacity of the blood may be reduced by the presence of carboxyhaemoglobin or methaemoglobin or, more rarely, by acute haemolysis. Cardiac output may be reduced by a number of poisons that cause cardiac arrhythmias, depress the contractility of the heart or cause extreme vasodilatation. The final step where poisons may interfere with the oxygen pathway is blockage of the cytochrome enzyme chain as occurs with toxins such as cyanide and hydrogen sulphide. These effects and the main poisons involved are summarized and outlined in Table 40.1. TABLE 40.1 Some typical causes of blockade of the oxygen pathway due to poisoning, with mechanisms and typical values In most cases of poisoning, the diagnosis is provided by the history and clinical examination. In some patients, however, there may be no history, though poisoning may be suspected because of the circumstances or the clinical features. In rare cases, the clinical presentation may mimic other medical conditions and it is necessary to consider the diagnosis of poisoning, especially when there are unusual features of the illness. Examples of these conditions include carbon monoxide, lead and paraquat poisoning, and drug and substance abuse. The clinical features of poisoning depend most of all on the type of agent involved, but also on the route of exposure (oral, intravenous, percutaneous or inhaled), the duration of exposure and time elapsed since exposure, and also the patient’s age and medical background (conditions such as diabetes, asthma, chronic renal failure and epilepsy). Clinical examination may reveal signs of injection marks, cutaneous burns, buccal corrosion, tablet residues in the mouth or cutaneous blisters. These blisters (originally called ‘barbiturate blisters’) may be found following overdose with barbiturates, benzodiazepines, tricyclic antidepressants or chloral hydrate, or exposure to carbon monoxide. There may be evidence of pulmonary aspiration or secondary hypostatic pneumonia in comatose patients. Clinical examination may indicate that a specific agonist/receptor pathway has been either over-stimulated or blocked by a poison. These syndromes are termed toxidromes and provide a useful initial guide to poison identification when the agent involved is unknown (Table 40.2). In the severely ill patient, baseline haematological and biochemical investigations will be necessary and arterial blood gas estimations will also be required. A chest radiograph is usually indicated. The results of haematological and biochemical investigations may be of great assistance in making the diagnosis and monitoring the response to treatment. The tests to be carried out and their frequency depend very much on the poison involved. Many of these are given later in this chapter, and a general summary is given in Table 40.3. Toxicological investigations may also be required: in some cases, these may only be available in specialized laboratories. The usual samples required are blood (note that whole blood is required for measurement of carboxyhaemoglobin, methaemoglobin, cyanide and several metals) and urine (50 mL in a universal container with no preservative). Urine should always be provided where possible, since many types of analysis, such as drugs of abuse, are more efficiently carried out on urine samples. Some of these assays are qualitative, but may be necessary in order to confirm the presence of compounds, which are not easily detectable in blood. Poisons centres now exist in most countries, with specialized facilities for provision of information on the toxicity of substances and on the management of poisoning. Most hold stocks of specialized antidotes, such as snake antivenoms. Many also have an analytical laboratory and some have patient care facilities. They are usually accessible by telephone or e-mail. In the UK, there are several poisons centres that provide information on poisoning: see Appendix 40.1. When an acutely ill patient presents, the first priority, regardless of the cause of the illness, is to establish the airway and provide immediate supportive treatment. Cardiopulmonary resuscitation or urgent ventilatory support may be needed. Depending on the patient’s state, questioning about the possible agent involved, obtaining an antidote or taking diagnostic samples may waste valuable time. In view of the frequency of opioid abuse, naloxone (an opioid antagonist) may have both a diagnostic and a therapeutic role. Once any immediate life-threatening problems have been dealt with, full supportive care must be provided while attempts are made to establish the diagnosis and treat the poisoning. In comatose patients, the most important measure is to maintain the airway and support ventilation as required, since respiratory complications are the commonest causes of death in unconscious poisoned patients. The unconscious patient should be placed in the left lateral (recovery) position in order to keep the airway patent and to minimize the risk of aspiration of gastric contents. Regular observation is essential as mechanical ventilation is not infrequently required. The circulation must be supported in order to maintain tissue perfusion and the electrocardiogram should be monitored, since cardiac dysrhythmias are common in poisoning. Antiarrhythmic agents and inotropic agents may be necessary. A brief convulsion due to cerebral hypoxia or to a toxic effect of the poison is not an indication for anticonvulsant drug therapy. However, if convulsions are repeated or prolonged, diazepam is the first-line drug. Drugs such as phenytoin, clomethiazole and thiopental may be required if there is no response to diazepam. In tricyclic antidepressant poisoning, administration of sodium bicarbonate may relieve convulsions. Phenytoin is contraindicated in tricyclic poisoning because both tricyclics and phenytoin are sodium channel blockers and these increase the risk of cardiac arrhythmias. The core temperature should be recorded with a low-reading thermometer, since hypothermia may complicate poisoning with sedative and antidepressant drugs. The patient should be wrapped in a space blanket, but active rewarming is not usually required. The prognosis is better than in accidental hypothermia from other causes, and temperatures as low as 22 °C are compatible with full recovery. In hyperthermia (rectal temperature > 39 °C), reduction of body temperature is a priority. Depending on the cause of the problem, different treatments may be required. Hyperthermic patients poisoned with agents that produce severe muscle rigidity may require elective paralysis with a muscle relaxant and mechanical ventilation. Malignant hyperthermia or neuroleptic malignant syndrome can be treated with intravenous dantrolene. Renal function should be monitored (bladder catheterization may be necessary if there is oliguria or severe hypovolaemia). Acute kidney injury may occur as a result of a direct toxic effect or secondary to acute haemolysis with haemoglobinuria or rhabdomyolysis with myoglobinuria. There is controversy as to whether myoglobin and haemoglobin are directly toxic to the kidneys. Most probably they cause an obstructive lesion; alkalinization of the urine increases myoglobin excretion and may prevent acute kidney injury. In the severely ill patient, general supportive care may be life saving. Regular turning will be needed to prevent pressure necrosis of tissues, and care of the eyes and mouth will also be required. Decontamination of the gut following ingestion of poisons is controversial. Syrup of ipecacuanha to induce emesis is now obsolete; there is little evidence that it is effective in emptying the stomach. In the comatose patient, gastric lavage is still occasionally used. However, this procedure may wash some of the poison further down the gut, and it is now rarely recommended. The most widely used method of gut decontamination is to administer activated charcoal, which adsorbs almost all drugs and poisons. The main exceptions are iron and lithium, which are poorly adsorbed, and alcohols and glycols, whose molar load usually exceeds the adsorptive capacity of the charcoal. Repeated doses of activated charcoal may be used for sustained-release preparations that have delayed absorption, and may also be used to eliminate certain poisons from the body if there is significant enterohepatic recycling (e.g. carbamazepine and theophylline). Antidotes form an important part of the management of certain poisons. In some cases (as with paracetamol, opioids, cardiac glycosides, organophosphates and snake bites), they may be life-saving. However, it should be noted that antidotal treatment is only required in a minority of poisonings. The most debated areas with regard to antidotes concern their effectiveness in ‘late’ paracetamol poisoning (when the patient presents more than 12 h after ingestion) and their effectiveness and use in the management of poisonings by metals. A list of commonly used antidotes is given in Table 40.4. In some types of poisoning, active elimination techniques are indicated. This approach is most widely used in poisoning by salicylates, theophylline, ethylene glycol and methanol. The techniques include repeated oral doses of activated charcoal, enhanced renal elimination (by alkalinization of the urine or diuresis), haemoperfusion and haemodialysis. Haemodialysis may be required for the elimination of the poison (most notably in the case of salicylates, ethylene glycol and methanol) or for the support of renal function. Exchange transfusion may be used in infants. Peritoneal dialysis, plasmapheresis and continuous arteriovenous haemofiltration are less useful in removing poisons, although the latter is an effective technique to support renal function. Although paracetamol is safe when taken in the recommended dose, it is potentially very toxic in overdose and, since it is widely available over the counter, it is currently the commonest cause of admissions to hospital for poisoning and also of acute hepatic necrosis. It causes approximately 150 deaths each year in Britain. Paracetamol is rapidly absorbed from the upper gastrointestinal tract and the majority is metabolized by conjugation with sulfate or glucuronate to non-toxic derivatives. However, a small proportion (approximately 8–10%) is metabolized by a specific cytochrome P450 enzyme, CYP2E1, to produce a highly reactive intermediate, N-acetyl-p-benzoquinoneimine (NAPQI). This reactive metabolite can be metabolized by conjugation to form non-toxic mercapturic acid conjugates, provided glutathione is present in the liver cell. When an overdose of paracetamol is taken, the rate of production of NAPQI may exhaust existing glutathione stores and the capacity of the liver to synthesize glutathione (Fig. 40.2). In this case, NAPQI covalently binds to sulfhydryl groups in hepatocytes, forming an irreversible complex, which can result in acute centrilobular necrosis of the liver. Since paracetamol is also metabolized in the cells of the renal tubules, a similar process can also occur in the kidneys, leading to acute kidney injury. There is usually a small amount of renal damage in the presence of hepatic damage, but occasionally renal damage predominates and acute kidney injury may rarely be the presenting feature of paracetamol poisoning. FIGURE 40.2 Metabolism of paracetamol. Induction of the cytochrome P450 pathway increases the production of the toxic metabolite, N-acetyl-p-benzoquinoneimine, causing depletion of glutathione stores; a protein-depleted diet leading to deficiency of amino acids may make less glutathione available. In both cases, increased toxicity may result following an overdose. The upper therapeutic limit for an adult of 4 g daily in divided doses is generally accepted as being safe, but doses above this can cause severe hepatotoxicity and possibly death. However, owing to the wide variation in the metabolic handling of paracetamol by the body, much larger overdoses (> 50 g) may have little effect in some individuals, producing nothing more than a minor rise in plasma aminotransferase activities. In the first hours after ingestion of an overdose of paracetamol, symptoms may be minimal, unless it has been taken with some other drug, for example in a compound formulation with dihydrocodeine. There may be malaise, nausea and vomiting. A very large overdose may cause depression of consciousness or metabolic acidosis. By 24–36 h, there may be pain in the right hypochondrium. Plasma aminotransferase activities will rise at this time, but the peak values are a poor indicator of prognosis. The prothrombin time (or international normalized ration, INR) is a reliable prognostic indicator of severe hepatotoxicity. Hypoglycaemia and coagulation defects may complicate the hepatic failure. By about 48 h, the early signs of hepatic encephalopathy may appear. Death usually occurs after five or six days. Giving an antidote is more important than emptying the stomach or giving activated charcoal. After a single acute overdose, an antidote should be given if the patient’s plasma paracetamol concentration is on or above the line on the treatment nomogram (Fig. 40.3). The need for treatment following repeated supratherapeutic dosing (staggered overdose) cannot be assessed using the nomogram and treatment decisions are based on the dose ingested and the patient’s body weight. Repeated plasma paracetamol estimations should not be performed routinely, but may be helpful in doubtful cases. Acetylcysteine is the antidote of choice and the intravenous route is the only reliable method of treatment in a patient who is comatose or vomiting. Although the effectiveness of acetylcysteine diminishes considerably with time once 12 h have passed since ingestion, later administration improves outcome even in patients with established liver failure. Acetylcysteine should also be given if the time of the overdose is in doubt or not known. Its main adverse effect is anaphylactoid reactions, which respond to administration of antihistamines and discontinuation or slowing of the infusion.
Poisoning
INTRODUCTION
AETIOLOGY OF POISONING
Intrauterine
Neonates
Infants
Childhood
Adult life
TYPES OF LESION IN POISONING
Note that whatever the initial effect of a poison, there may be subsequent progression down the list of effects towards cell death. The effects of two or more poisons may be additive
Cause
Mechanism
Effect
Asphyxiant gases (e.g. butane, methane, carbon dioxide, nitrogen)
Hypoxic gas mixture
Reduced inspired oxygen fraction (FiO2) (normal ~ 0.21)
Respiratory depression (e.g. opioids, barbiturates, other sedatives and hypnotics). Respiratory muscle disorders: paralysis (e.g. organophosphates, botulinum toxin) or spasm (e.g. strychnine, phencyclidine)
Failure of ventilation (type II respiratory failure)
Reduced alveolar oxygen tension (PAO2) (normal ~ 13.3 kPa)
Aspiration pneumonitis
Adult respiratory distress syndrome (e.g. paraquat)
Failure of oxygen transfer (type I respiratory failure)
Reduced arterial oxygen tension (PaO2) (normal 10–13.3 kPa)
Carboxyhaemoglobin (carbon monoxide); methaemoglobin (e.g. nitrites); haemolysis (e.g. arsine, stibine)
Loss of functioning haemoglobin
Reduced arterial oxygen content (CaO2) (normal 18–21 volumes %)
Myocardial depressants (e.g. β-blockers, calcium antagonists, tricyclic antidepressants, dextropropoxyphene, cocaine)
Reduced cardiac output
Reduced tissue oxygen delivery (QO2) (normal 12–16 mL/kg per min)
Chemical asphyxiants (cyanide, hydrogen sulphide)
Block of cytochrome enzyme chain
Reduced tissue oxygen consumption (VO2) causing failure of oxidative metabolism (normal 3–4 mL/kg per min). Cell death
DIAGNOSIS AND MANAGEMENT OF POISONING: GENERAL PRINCIPLES
Diagnosis
Management
Respiratory support
Cardiovascular support
Central nervous system complications
Body temperature
Renal complications
General supportive care
Intestinal decontamination
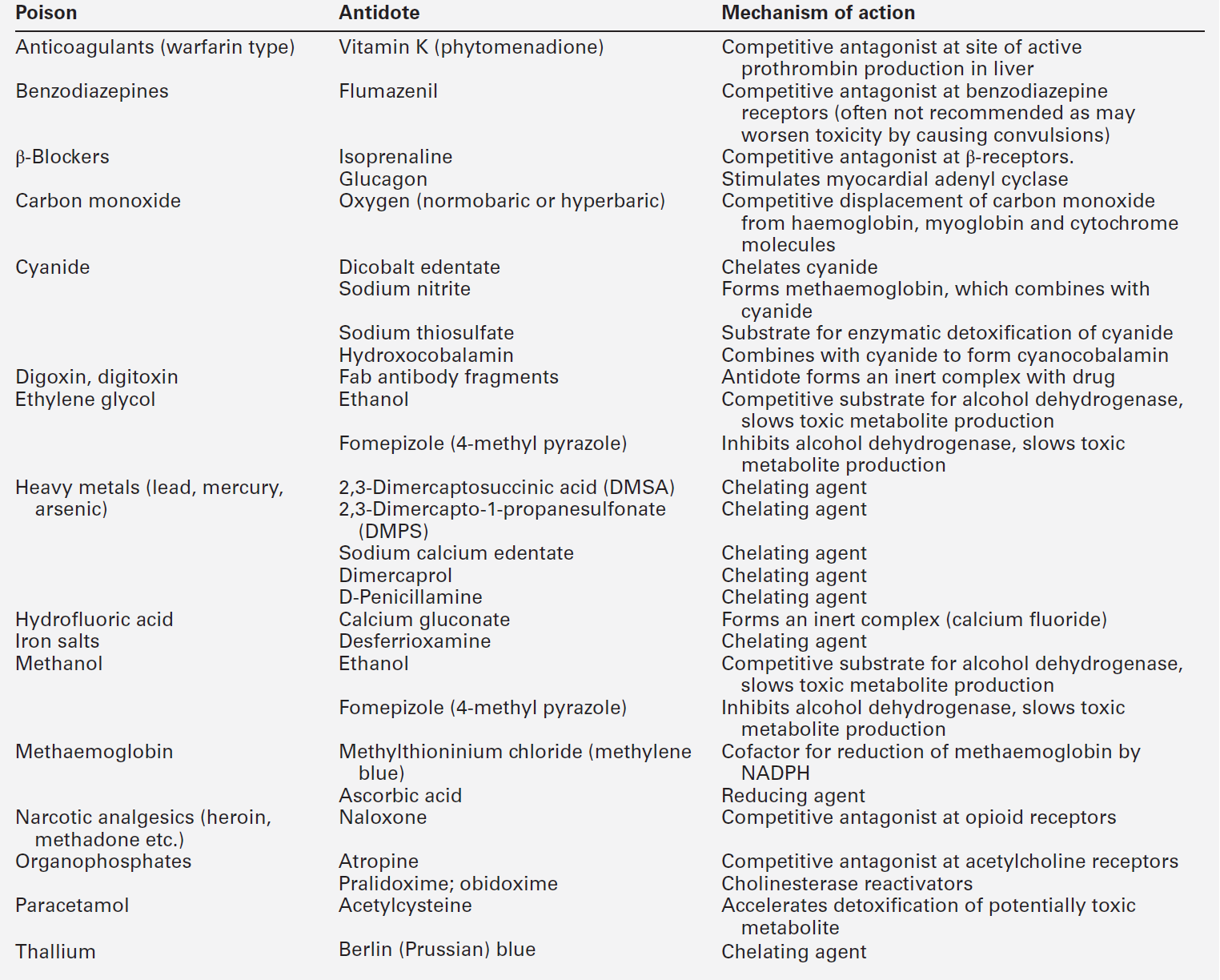
Antidotes
Elimination techniques
SPECIFIC POISONS
Paracetamol (acetaminophen)
Mechanisms
Toxic dose
Clinical features
Management
Salicylate
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree