EMBRYOLOGY OF THE GENITOURINARY TRACT
A basic understanding of genitourinary embryology facilitates learning many aspects of urology. Embryologically, the genital and urinary systems are intimately related. Associated anomalies of the two systems are commonly encountered.
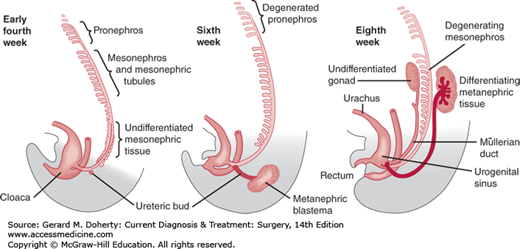
The kidneys pass through three embryonic phases (Figure 38–1): (1) The pronephros is a vestigial structure without function in human embryos that, except for its primary duct, disappears completely by the fourth week. (2) The pronephric duct gains connection to the mesonephric tubules and becomes the mesonephric duct. While most of the mesonephric tubules degenerate, the mesonephric duct persists bilaterally; from where it bends to open into the cloaca, the ureteral bud grows cranially to interact with the metanephric blastema. (3) This forms the metanephros, which is the final phase. The metanephros develops into the kidney. During cephalad migration and rotation, the metanephric tissue progressively enlarges, with rapid internal differentiation into the nephron and the uriniferous tubules. Simultaneously, the cephalad end of the ureteral bud expands and divides within the metanephros to form the renal pelvis, calices, and collecting tubules.
Figure 38–1.
Schematic of the development of the nephric system. Only a few of the tubules of the pronephros are seen early in the fourth week, while the mesonephric tissue differentiates into mesonephric tubules that progressively join the mesonephric duct. The first sign of the ureteral bud from the mesonephric duct is seen at 4 weeks. At 6 weeks, the pronephros has completely degenerated and the mesonephric tubules start to do so. The ureteral bud grows dorsocranially and has met the metanephric blastema. At the eighth week, there is cranial migration of the differentiating metanephros. The cranial end of the ureteral bud expands and starts to show multiple successive outgrowths (renal calices).
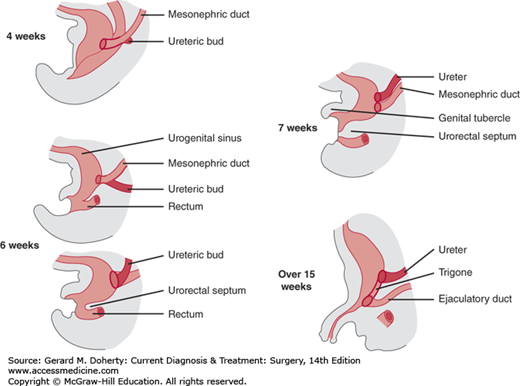
Subdivision of the cloaca (the blind end of the hindgut) into a ventral (urogenital sinus) and a dorsal (rectum) segment is completed during the seventh week and initiates early differentiation of the urinary bladder and urethra. The urogenital sinus receives the mesonephric duct and absorbs its caudal end, so that by the end of the seventh week, the ureteral bud and mesonephric duct have independent openings. The ureteral orifice migrates upward and laterally. The mesonephric duct orifice moves downward and medially, and the structure in between (the trigone) is formed by the absorbed mesodermal tissue, which maintains direct continuity between the two tubes (Figure 38–2).
Figure 38–2.
The development of the ureteral bud from the mesonephric duct and their relationship to the urogenital sinus. The ureteral bud appears at the fourth week. The mesonephric duct distal to this ureteral bud is gradually absorbed into the urogenital sinus, resulting in separate endings for the ureter and the mesonephric duct. The mesonephric tissue that is incorporated into the urogenital sinus expands and forms the trigonal tissue. The mesonephric duct forms the vas deferens in the male and Gartner’s duct (if present) in the female.
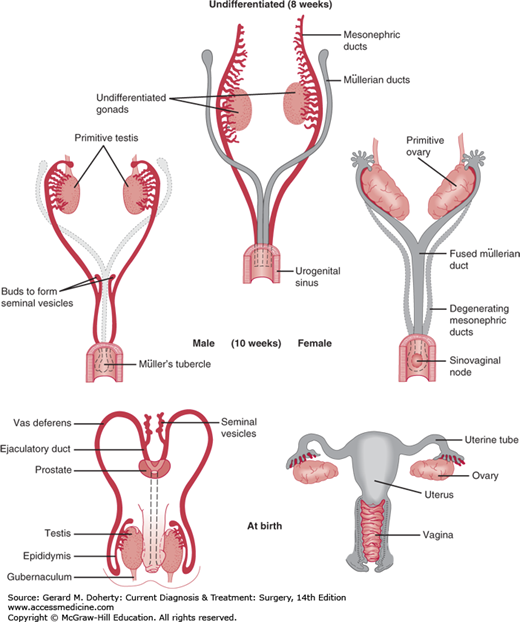
The fused müllerian ducts also meet the urogenital sinus at Müller’s tubercle. The urogenital sinus above Müller’s tubercle differentiates to form the bladder and the part of the prostatic urethra proximal to the seminal colliculus in the male or the bladder and the entire urethra in the female (Figure 38–3). Below Müller’s tubercle, the urogenital sinus differentiates into the distal part of the prostatic urethra and the membranous urethra in the male or the distal vagina and vaginal vestibule in the female. The rest of the male urethra is formed by fusion of the urethral folds on the ventral surface of the genital tubercle. In the female, the genital folds remain separate and form the labia minora.
The prostate develops at the end of the 11th week as several groups of outgrowths of urethral epithelium both above and below the entrance of the ejaculatory duct (distal vas deferens). The developing glandular element (seminal colliculus) incorporates the differentiating mesenchymal cells surrounding it to form the muscular stroma and capsule of the prostate. The seminal vesicles form as duplicate buds from the distal end of the mesonephric duct (vas deferens).
The potential to differentiate along male or female lines is present in every embryo initially. The development of one set of sex primordia and the gradual involution of the other are determined by the genetic sex of the embryo and differential secretion of numerous hormones. The SRY gene—or testis-determining factor—on the Y chromosome drives the gonad to differentiate into a testicle. Gonadal differentiation begins during the seventh week (Figure 38–3). If the gonad develops into a testis, the germinal epithelium progressively grows into radially arranged, cord-like seminiferous tubules. The production of müllerian-inhibiting factor by the testicle causes regression of the müllerian duct and acts in a local (paracrine) fashion so that only the ipsilateral müllerian duct is affected. The subsequent production of testosterone by the testicle leads to masculinization of the mesonephric (wolffian) duct structures (ie, epididymides, vas deferens, seminal vesicles, and ejaculatory duct). If the gonad develops into an ovary, it becomes differentiated into a cortex and a medulla; the cortex later differentiates into ovarian follicles containing ova. The lack of testosterone leads to the disappearance of the mesonephric duct.
The testes remain in the abdomen until the seventh month and then pass through the inguinal canal to the scrotum, following the path of the gubernaculum. The mechanism of descent remains uncertain. Lack of complete testicular descent is known as cryptorchidism; descent to an abnormal site beyond the external inguinal ring is known as testicular ectopia.
The ovary, which is attached to ligaments, undergoes internal descent to enter the pelvis.
In the female, the genital duct system develops from the müllerian ducts, which fuse at their caudal ends and differentiate into the uterine tubes, the uterus, and the proximal two-thirds of the vagina.
The external genitalia start to differentiate by the eighth week. The genital tubercle and genital swellings develop into the penis and scrotum in the male and the clitoris and labia majora in the female. The external genitalia are masculinized by dihydrotestosterone (DHT), which is created from testosterone under the influence of 5α-reductase.
With the breakdown of the urogenital membrane in the seventh week, the urogenital sinus achieves a separate opening on the undersurface of the genital tubercle. The expansion of the infratubercular part of the urogenital sinus forms the vaginal vestibule and the distal third of the vagina. The two folds on the undersurface of the genital tubercle unite in the male to form the penile urethra; in the female, they remain separate to form the labia minora.
ANATOMY OF THE GENITOURINARY TRACT: GROSS & MICROSCOPIC
The kidneys lie retroperitoneally in the posterior abdomen and are separated from the surrounding renal fascia (Gerota’s fascia) by perinephric fat. The renal vascular pedicle enters the renal sinus; the vein is anterior to the artery, and both are anterior to the renal pelvis. The renal artery divides just outside the renal sinus into anterior and posterior branches that undergo further subdivisions with variable extents of distribution. They are end arteries and thus result in segmental infarction when occluded. The venous tributaries anastomose freely and usually drain into one renal vein.
The renal parenchyma consists of more than 1 million functioning units (nephrons) and is divided into a peripheral cortex containing secretory elements and a central medulla containing excretory elements. The nephron starts as Bowman’s capsule, which surrounds the glomerulus and leads to elongated proximal and distal convoluted tubules with the loop of Henle in between, ending in a collecting duct that opens into a minor calix at the tip of a papilla.
The renal pelvis and calices are within the renal sinus and function as the main collecting reservoir. The pelvis, which is partly extrarenal and partly intrarenal (but occasionally is totally extrarenal or intrarenal), branches into three major calices that in turn branch into several minor calices. These calices are directly related to the tips of the medullary pyramids (the papillae) and act as a receiving cup to the collecting tubules. The pelvicaliceal system is a highly muscular structure; the fibers run in many directions and are directly continuous from the calices to the pelvis, allowing synchronization of contractile activity.
The ureter connects the renal pelvis to the urinary bladder. It is a muscularized tube; its muscle fibers lie in an irregular helical arrangement and function primarily in peristaltic activity. Ureteral muscle fibers are directly continuous from the renal pelvis cranially to the vesical trigone distally.
The blood supply to the renal pelvis and ureters is segmental, arising from multiple sources, including the renal, gonadal, and vesical arteries, with rich subadventitial anastomoses.
The bladder is primarily a reservoir with a meshwork of muscle bundles that not only change from one plane to another but also branch and join each other to constitute a synchronized organ. Its musculature is directly continuous with the urethral musculature and thus functions as an internal urethral sphincteric mechanism in spite of the lack of a true circular sphincter.
The ureters enter the bladder posteroinferiorly through the ureteral hiatus; after a short intravesical submucosal course, they open into the bladder and become continuous with the trigone, which is superimposed on the bladder base though deeply connected to it.
The adult female urethra is about 4 cm long and is muscular in its proximal four-fifths. This musculature is arranged in an inner longitudinal coat that is continuous with the inner longitudinal fibers of the bladder and an outer circular coat that is continuous with the outer longitudinal coat of the bladder. These outer circular fibers comprise the sphincteric mechanism. The striated external sphincter surrounds the middle third of the urethra.
In the male, the prostatic urethra is heavily muscular and sphincteric. The membranous urethra is within the urogenital diaphragm and is surrounded by the striated external sphincter. The penile urethra is poorly muscularized and traverses the corpus spongiosum to open at the tip of the glans.
The prostate surrounds the proximal portion of the male urethra; it is a fibromuscular, cone-shaped gland about 2.5 cm long and normally weighing about 20 g in the adult. It is traversed from base to apex by the urethra and is pierced posterolaterally by the ejaculatory ducts from the seminal vesicles and vas deferens that converge to open at the verumontanum (seminal colliculus) on the floor of the urethra.
The prostatic glandular elements drain through about 12 paired excretory ducts that open into the floor of the urethra above the verumontanum. The prostate is surrounded by a thin capsule, derived from its stroma, which is rich in musculature, and part of the urethral musculature and the sphincteric mechanism. A rich venous plexus surrounds the prostate, especially anteriorly and laterally. Its lymphatic drainage is into the hypogastric, sacral, obturator, and external iliac lymph nodes.
The testis is a paired organ surrounded by the tunica albuginea and subdivided into numerous lobules by fibrous septa. The extremely convoluted seminiferous tubules gather to open into the rete testis, where they join the efferent duct and drain into the epididymis. The epididymis drains into the vas deferens, which courses through the inguinal canal into the pelvis and is joined by the duct from the seminal vesicle to form the ejaculatory duct, which opens before opening into the prostatic urethra on either side of the verumontanum.
Arterial supply is via the spermatic, vas deferential, and external cremasteric arteries. Venous drainage is through the pampiniform plexus, which drains into the internal spermatic veins; the right spermatic vein joins the vena cava, and the left joins the renal vein.
Testicular lymphatics drain into the retroperitoneal lymph nodes; the right primarily into the interaortocaval area, the left into the para-aortic area, both just below the renal vessels.
PHYSIOLOGY OF THE GENITOURINARY TRACT
The kidneys maintain and regulate homeostasis of body fluids by glomerular filtration, tubular reabsorption, and tubular secretion.
This mechanism is dependent on glomerular capillary arterial pressure minus plasma colloid osmotic pressure plus Bowman’s capsular resistance. The resultant glomerular filtration pressure (about 8-12 mm Hg) forces protein-free plasma through the capillary filtering surface into Bowman’s capsule. Normally, about 130 mL of plasma is filtered every minute through the renal circulation; the entire volume of plasma recirculates through the kidney and is subjected to the filtration process once every 27 minutes.
About 99% of the filtered volume is reabsorbed through the tubules, together with all the valuable constituents of the filtrate (chlorides, glucose, sodium, potassium, calcium, and amino acids). Urea, uric acid, phosphates, and sulfates are also reabsorbed to varying degrees. The process of reabsorption is a combination of active and passive transport mechanisms. Reabsorption of water and electrolytes is under the control of adrenal, pituitary, and parathyroid hormones.
Tubular secretion helps (1) to eliminate certain substances and thus maintain their plasma levels and (2) to exchange valuable ions from the filtrate for less desirable ions in the plasma (eg, a sodium ion from the urine for a hydrogen ion in the plasma). Failure of adequate secretory function leads to the acidosis commonly encountered in chronic renal disease.
This system is one continuous tubular structure with a syncytial type of smooth musculature that is imperceptibly in motion from one segment to the other. Waves of peristaltic contractions start from the calices and are propagated along the smooth muscle cells to the renal pelvis. At normal urine flow rates, many of these contraction waves are terminated at the ureteropelvic junction; however, some are transmitted to the ureter and down toward the urinary bladder. These peristaltic waves occur at a rate of about 5-8/min, involve a 2-cm to 3-cm segment at a time, and usually proceed at the velocity of 3 cm/s. Frequency, amplitude, and velocity are influenced by urine output and flow rate. In a state of diuresis, there may be a 1:1 relationship between caliceal contractions and ureteral contractions. Ureteral filling is primarily passive and occurs by reception of a bolus of urine from a renal pelvis contraction. The ureteropelvic junction closes after passing a bolus of urine, preventing back-pressure and back-flow of urine into the renal pelvis secondary to the elevated ureteral contraction pressure. A contraction ring forms in the proximal ureter, and as it migrates down the ureter, it pushes the bolus of urine antegrade. In states of diuresis, the size of the bolus increases and the pressure in the bolus may be greater than the pressure in the contraction ring ahead of it. In this case, the ureteral walls cannot coapt, and urine is transported as an uninterrupted column of fluid.
The ureterovesical junction allows flow of urine from the ureter to the bladder and at the same time prevents retrograde flow. The continuity and the specific muscular arrangement of the intravesical ureter and the trigone provide a muscularly active valvular mechanism that can efficiently adapt itself to the variable phases of bladder activity during filling and voiding.
The normal resting pressure of the ureterovesical junction (10-15 cm H2O) is greater than the more cephalad ureteral resting pressure (0-5 cm H2O). Progressive bladder filling leads to firm occlusion of the intravesical ureter against retrograde urine flow and to increased resistance to antegrade flow resulting from trigonal stretching. During voiding, trigonal contraction completely seals the intravesical ureter against any antegrade or retrograde flow of urine.
The urinary bladder functions primarily as a reservoir that can accommodate variable volumes without increasing its intraluminal pressure. When the bladder reaches full capacity, the detrusor muscle voluntarily contracts following relaxation of the external sphincter and maintains its contraction until the bladder is completely empty. Funneling of the bladder outlet with progressive downward movement of the dome ensures complete emptying.
The vesical sphincteric mechanism is primarily a smooth muscle sphincter in the bladder neck and male prostatic urethra and in the proximal four-fifths of the female urethra. There is no purely circular sphincteric entity, but there are abundant circularly oriented smooth muscle fibers that are directly continuous with the outer coat of the detrusor muscles. The sphincter has an abundance of alpha receptors that respond to sympathetic neural input from the pelvic nerve to maintain urethral closure. Parasympathetic input from the pelvic nerve facilitates bladder contracture and voiding.
There is a voluntary striated muscle sphincter that is part of the urogenital diaphragm and surrounds the mid urethra in the female and the membranous urethra in the male. It responds to somatic neural input from the pudendal nerve. It is essential for continence when the internal sphincter is nonfunctional. Its pathologic irritability or spasticity can lead to obstructive manifestations.
DEVELOPMENTAL ANOMALIES OF THE GENITOURINARY TRACT
Genitourinary tract anomalies constitute about one-third of all congenital abnormalities and occur in over 10% of the population. The severity varies from lesions incompatible with life to insignificant findings detected during diagnostic studies for unrelated reasons. The anatomic abnormalities are often not intrinsically harmful, yet they may predispose to infection, stone formation, or chronic renal failure.
Bilateral absence of the kidneys is rare and is associated with oligohydramnios, Potter facies, and pulmonary hypoplasia. It occurs more often in males and results in death shortly after birth. Unilateral renal agenesis is seen more often but is not usually associated with illness. Renal agenesis is thought to be due to both lack of a ureteral bud and lack of subsequent development of the metanephric blastema. The trigone is absent on the affected side. Because adrenal gland development is unrelated to kidney development, both adrenals are usually present in the normal position. Rarely, more than two kidneys are seen, a condition clearly dissimilar to ureteral duplication, as described later.
Abnormal ascent of the metanephros leads to an ectopic kidney, which may be unilateral or bilateral. Lumbar, pelvic, and the less common thoracic and crossed ectopic varieties are seen. Ectopic kidneys are associated with genital anomalies in 10%-20% of cases. Fusion abnormalities are also associated with failure of normal ascent and include fused pelvic kidneys and horseshoe kidneys (the most common), which are typically fused at the lower poles. Intravenous urography typically establishes the diagnosis. The relationship of the kidneys to the psoas muscles is abnormal: Instead of an oblique orientation with the medial border of the kidney parallel to the psoas muscle, the kidneys are vertical and the medial border intersects and crosses the psoas muscle. Horseshoe kidneys have an elevated incidence of vesicoureteral reflux and are at increased risk of ureteropelvic junction obstruction (UPJO). The latter may be related to a high ureteral insertion in the renal pelvis, crossing of the ureter over the isthmus, or compression by one of many anomalous arteries. Failure of rotation during ascent results in “malrotated” kidneys and is rarely significant.
Parenchymal anomalies include a variety of cystic and dysplastic lesions. Polycystic kidney disease is hereditary and bilateral. The autosomal recessive polycystic kidney disease (ARPKD), previously called infantile PKD, has numerous small cysts that arise only from the collecting ducts and result in bilateral symmetrical enlargement of the kidneys. The autosomal dominant ADPKD, previously called adult PKD, has cysts arising from all areas of the nephron, which are usually larger and more variable in size than the ARPKD cysts. ARPKD occurs in 1 in 40,000 births and may be detected in utero by the presence of enlarged hyperechogenic kidneys and oligohydramnios. Infants usually die of respiratory failure rather than renal problems; however, the 1-year survival probability after the first month is over 85%. These children have declining renal function as well as severe hypertension and hepatic periportal fibrosis with portal hypertension leading to hypersplenism and esophageal varices.
The genes mutated in ADPKD may include the PKD1 gene (located on chromosome 16p13.3) in 85% of patients or the PKD2 gene (on chromosome 4q21-23) in 12%-15% of patients. These genes code for the polycystin-1 and polycystin-2 proteins, respectively. ADPKD occurs in 1 in 1000 individuals and is a major cause of end-stage renal disease in adults. Cysts may also be present in the liver, pancreas, and spleen, and cerebral arterial aneurysms may occur. Renal cystic enlargement exerts pressure on normal parenchyma, leading to its gradual destruction and glomerulosclerosis.
The diagnosis is often made during a workup for hypertension or uremia discovered in the third to sixth decades. Hematuria with or without flank pain is a common finding. An intravenous urogram reveals the enlarged kidneys, with marked elongation of the calices, which are compressed by large cysts. Ultrasonography or CT scan readily makes the diagnosis.
Surgery is rarely warranted. Therapy is medical and ultimately includes dialysis. The median age for reaching end-stage renal disease is 54 years in PKD1 and 74 years in PKD2. Renal transplantation is often indicated, though potential family donors must be carefully screened to determine whether they have the same disorder. The leading cause of death in ADPKD is cardiovascular disease, which may relate to early untreated hypertension.
Medullary sponge kidney results from collecting tubular ectasia (see section on Polycystic Kidneys) and is associated with recurrent urolithiasis and an increased incidence of infection in 50% of patients. The lesion is often bilateral and may involve all of the calices. Intravenous urograms reveal dilated collecting tubules as a “blush” in the renal papilla. Microscopic hematuria is common. Specific antibiotics should be given for documented infections, and prophylactic therapy for renal stones should be recommended on the basis of metabolic stone evaluation.
Simple renal cysts are common (approximately 50% after age 50) and are thought to arise from tubular dilation. They may be solitary or bilateral and multiple. They rarely have pathologic significance except in the differentiation from solid renal masses. (See the section on Renal Adenocarcinoma.)
Multicystic dysplastic kidney is a congenital abnormality consisting of macroscopic cysts of variable sizes compressing dysplastic renal parenchyma. It is usually associated with an atretic proximal ureter. The disorder occurs in about 1 in 3000 live births and is frequently noted on prenatal ultrasound. Rarely, it may occur bilaterally and is associated with oligohydramnios and renal failure. It may be distinguished from other causes of hydronephrosis by the absence of any renal function on renal scan. There is an increased incidence of contralateral UPJO (5%-10%) and reflux (18%-43%), either of which increases the patient’s risk of subsequent chronic renal insufficiency.
The chance of developing a malignancy in multicystic dysplastic kidney appears to be no greater than 1 in 2000. There may also be an increased incidence of hypertension. These two factors constitute a rationale for treatment by nephrectomy. However, conservative management with routine ultrasound examinations at intervals of 6-12 months is reasonable practice, since about half involute within 5 years.
Multiple renal arteries occur in 15%-20% of patients and are significant only when they cause UPJO. Congenital renal artery aneurysms are infrequent; they are differentiated from acquired lesions by their location at the bifurcation of the main renal artery or at a distal branch point. The lesions are usually asymptomatic, but they can cause hypertension. They require surgical treatment only if hypertension is uncontrolled, if they are incompletely calcified, or if they have a diameter of more than 2.5 cm. Congenital arteriovenous fistulas are rare but may result in hematuria, hypertension, or cardiac failure necessitating operative treatment.
UPJO is the most common cause of antenatal hydronephrosis. The condition may be associated with compression by anomalous renal arteries or intrinsic stenosis of the junction. The diagnosis is not uncommonly made when gross hematuria follows minor trauma. Symptoms include intermittent flank pain, particularly with orally induced diuresis. There is a bimodal age at presentation, with an initial peak in infancy and a secondary presentation in early adulthood. Renal ultrasound provides a safe screening technique in patients suspected of having UPJO. Diuretic renal scan may confirm the diagnosis and suggest functional significance. Intravenous pyelogram or retrograde pyelography may further define the anatomy. Bilaterality is not uncommon, and the condition requires surgical repair if symptomatic or severe. With the advent of laparoscopic and robotic surgery, minimally invasive repair of UPJO with dismemebered pyeloplasty has become standard of care. Success rates with pyeloplasty are superior to endoscopic approaches in the primary setting. Percutaneous or ureteroscopic incision of the obstruction with short-term stenting has been successful in adults, but endoscopic incision is most useful in the setting of recurrent UPJO, when redo pyeloplasty becomes much more difficult, or when patients are poor surgical candidates due to comorbidities.
Congenital obstruction of the ureter may be due to ureterovesical and UPJO or to neurologic deficits such as sacral agenesis or myelomeningocele. Functional ureteral obstruction—also known as primary obstructive megaureter—is not uncommon. Symptoms are renal pain during diuresis or resulting from pyelonephritis. Excretory urograms depict dilation above the obstruction. Vesicoureteral reflux is uncommonly associated with megaureter. Milder forms without symptoms or significant hydronephrosis are the rule and do not require treatment if renal function is normal. When treatment is necessary, it consists of division of the ureter proximal to the obstruction and reimplantation of the ureter into the bladder, often involving ureteral tapering or plication.
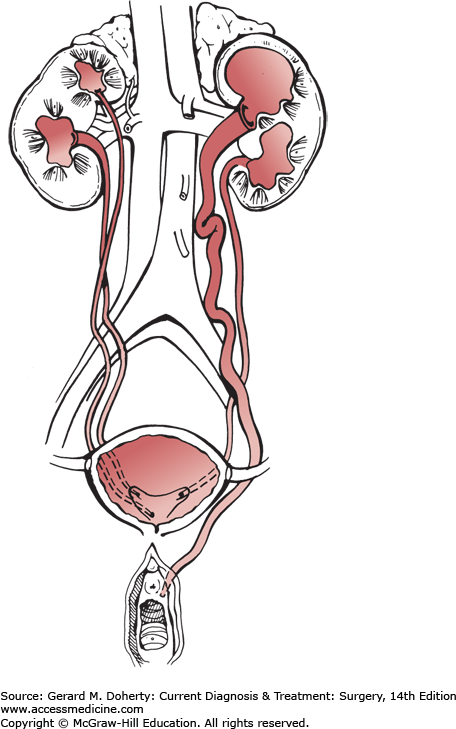
Bifurcation of the ureteral bud before it interacts with the metanephric blastema results in incomplete ureteral duplication, commonly in the mid or upper ureter. A second ureteral bud from the metanephric duct leads to complete ureteral duplication (Figure 38–4; right kidney) draining one kidney. This represents the most common ureteral anomaly, occurring in 1 in 125 people. It occurs twice as often in females. The presence of more than two ureters on each side is not common, but bilaterality of ureteral duplication occurs in 40%. Usually, all of the duplicated ureters enter the bladder; the ureter draining the upper pole of the kidney enters closest to the bladder neck (due to its later reabsorption into the bladder). Because of this relationship, the ureter draining the lower pole often has a short intramural tunnel and an inadequate surrounding musculature and is thus prone to vesicoureteral reflux. The ureter draining the upper pole may be ectopic (because of its late absorption) and thus empty into the bladder neck, urethra, or genital structures (vagina or vestibule in the female and seminal vesicle or vas deferens in the male [Figure 38–4; left kidney]). The ureter draining the upper pole is prone to obstruction and may be associated with a ureterocele, which is a common cause of obstruction. Duplication becomes significant when hydronephrosis or pyelonephritis occurs. The diagnosis is made by intravenous urography. Ureteral reimplantation to prevent recurrent infection is necessary in some cases. An anastomosis between the upper pole renal pelvis and the lower pole ureter or a low ureteroureterostomy are alternatives in selected cases. The upper pole of the kidney and its ureter may require removal if obstruction is severe and renal function of that segment is poor.
Ureteral ectopia can occur in the absence of duplication and drain into any of the abnormal positions mentioned previously. If the orifice lies proximal to the external urinary sphincter, no incontinence ensues, but vesicoureteral reflux is common. In contradistinction to the female, the ectopic orifice in the male never lies distal to the external sphincter, making incontinence an extremely rare presentation. Should the ectopic orifice in the female drain into the vagina or at the vestibule, there may be continuous leakage of urine apart from voiding. Most ectopic orifices involve the ureter draining the upper pole of a duplicated system, and most are observed in females. Hydroureteronephrosis of the involved segment frequently occurs due to ureteral obstruction as it traverses the muscle of the bladder neck.
An ectopic orifice may be seen beside the urethral orifice or in the roof of the vagina on endoscopy. Renal ultrasound or intravenous urograms often demonstrates hydroureteronephrosis of the upper renal segment. Cystography may show reflux into the ectopic orifice but may require cyclic voiding first to decompress the obstructed segment with bladder neck relaxation and subsequently to permit reflux. In the rare case when there is significant upper pole renal function, the ureter can be divided and reimplanted into the bladder or lower pole ureter. Usually, however, heminephroureterectomy is necessary.
A ureterocele is a ballooning of the distal submucosal ureter into the bladder. This structure commonly has a pinpoint orifice and therefore leads to hydroureteronephrosis. If large enough, it may obstruct the vesical neck or the contralateral ureter. It is most common in females with ureteral duplication and always involves the ureter draining the upper renal pole.
Most ureteroceles are now detected by prenatal ultrasound. Symptoms are usually those of pyelonephritis or obstruction. Intravenous urograms may show a negative shadow in the bladder cast by the ureterocele. The ureter and renal calices may be normal or may reveal marked dilation or no excretory function at all. A cystogram may show reflux into the ipsilateral lower pole ureter.
Treatment of ureteroceles depends on multiple factors, including the presence or absence of reflux in any or all of the ureters as well as whether or not the ureterocele is completely contained within the bladder (intravesical/orthotopic) or if a portion is at the bladder neck or urethra (extravesical/ectopic). A simple method of establishing drainage involves cystoscopy and puncture of the ureterocele. Associated reflux, if present, can be managed with prophylactic antibiotics until the child has grown larger, at which time a technically easier ureteral reimplant may be performed with a decompressed ureter. In the relatively uncommon situation when there is no associated reflux, an upper pole heminephrectomy is considered. Minimally obstructive ureteroceles within the bladder in adults do not require treatment.
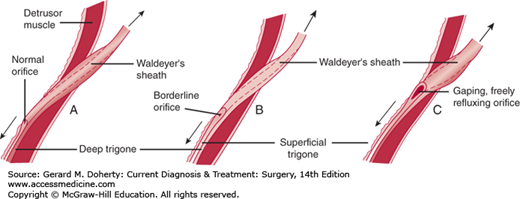
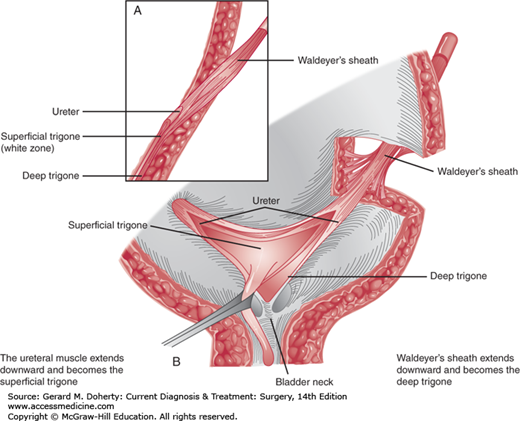
The main function of the ureterovesical junction is to permit free drainage of the ureter and simultaneously prevent urine from refluxing back from the bladder. Anatomically, the ureterovesical junction is well equipped for this function, because the ureteral musculature continues uninterrupted into the base of the bladder to form the superficial trigone. Additionally, the terminal 4-5 cm of ureter are surrounded by a musculofascial sheath (Waldeyer’s sheath) that follows the ureter through the ureteral hiatus and continues in the base of the bladder as the deep trigone (Figure 38–5).
Direct continuity between the ureter and the trigone offers an efficient, muscularly active, valvular function. Any stretch of the trigone (with bladder filling) or any trigonal contraction (with voiding) leads to firm occlusion of the intravesical ureter, thus increasing resistance to flow from above downward and sealing the intravesical ureter against retrograde flow (Figure 38–6).
Figure 38–5.
Vesicoureteral reflux. The length and fixation of the intravesical ureter and the appearance of the ureteral orifice depend on the muscular development and efficiency of the lower ureter and its trigone. A: Normal structures. B: Moderate muscular deficiency. C: Marked deficiency results in a golf hole distortion of the submucosal ureter.
Figure 38–6.
Normal ureterotrigonal complex. A: Side view of ureterovesical junction. The Waldeyer muscular sheath invests the juxtavesical ureter and continues downward as the deep trigone, which extends to the bladder neck. The ureteral musculature becomes the superficial trigone, which extends to the verumontanum in the male and stops just short of the external meatus in the female. B: Waldeyer’s sheath is connected by a few fibers to the detrusor muscle in the ureteral hiatus. This muscular sheath, inferior to the ureteral orifices, becomes the deep trigone. The musculature of the ureters continues downward as the superficial trigone. (Adapted, with permission, from Tanagho EA, Pugh RCB: The anatomy and function of the ureterovesical junction. Br J Urol. 1963 June;35(2):151–165.)
Vesicoureteral reflux may be classified as primary reflux due to developmental ureterotrigonal weakness or associated with ureteral anomalies such as ectopic orifice or ureterocele, and secondary reflux due to bladder outlet or urethral obstruction, neuropathic dysfunction, iatrogenic causes, and inflammation, especially specific infection (eg, tuberculosis). Primary reflux is associated with some degree of congenital muscular deficiency in the trigone and terminal ureter.
Reflux is associated with an increased incidence of pyelonephritis and renal damage. It also allows bacteria free access from the bladder to the kidney.
Reflux is the most common cause of pyelonephritis and is found in 30% to 50% of children presenting with urinary tract infection. It is present in over 75% of patients with radiologic evidence of chronic pyelonephritis and is responsible for end-stage renal disease in a large percentage of patients requiring chronic dialysis or renal transplantation.
In primary reflux, the child (on average, between 2 and 3 years of age) usually presents with symptoms of pyelonephritis or cystitis. Vague abdominal pain is not uncommon. Renal pain and pain with voiding are relatively uncommon. On rare occasions, the patient may present with advanced renal failure with bilateral renal parenchymal damage. Significant reflux and its sequelae are more common in females and are usually detected after a urinary tract infection. About one-third of the siblings of a child with reflux will also have reflux, and one-half of the children of a mother with reflux will also have reflux.
In secondary reflux, manifestations of the primary disease (neuropathic, obstructive, etc) are usually the presenting symptoms.
With acute pyelonephritis, fever, chills, and costovertebral angle tenderness may be present. Children usually do not have renal pain but may complain of vague abdominal pain. Occasionally, daytime frequency, incontinence, or enuresis may be caused by infection associated with reflux. In cases of obstruction or neuropathic deficit, a palpable hydronephrotic kidney or a distended bladder may be found. The diagnosis may be elusive in infants who present with ill-defined symptoms.
Urinalysis usually reveals evidence of infection (pyuria and bacteriuria). Urine cultures are mandatory when infection is suspected. Renal function tests may be abnormal if reflux and infection have caused renal scarring.
The most useful study for conclusive diagnosis of reflux continues to be voiding cystourethrography (Figure 38–7). This study demonstrates the grade of reflux as well as the urethral anatomy. Radionuclide voiding studies are extremely sensitive at detecting reflux but do not demonstrate the anatomic detail seen with a voiding cystourethrogram. Radionuclide voiding studies are often performed as follow-up after an initial voiding cystourethrogram because they offer the advantage of decreased radiation exposure.
Radioisotopic renal scanning provides accurate differential renal function data and detection of renal scars. Ultrasound can provide accurate measurement of renal size and may demonstrate the presence of renal scarring and ureteral or caliceal dilation. In many cases, there may be no abnormality visible in the upper urinary tract, or only mild distal ureteral dilation may be seen.
A significant number of children with dysfunctional voiding present with urinary tract infections and are subsequently found to have reflux. These children contract the bladder against a closed external sphincter. Elevated voiding pressures associated with dysfunctional voiding may increase renal damage with an associated urinary tract infection and may also lessen the chance for either spontaneous or surgical resolution of reflux. When the history suggests the possibility of voiding dysfunction (incontinence, frequency, urgency), urodynamic studies are conducted to evaluate the voiding dynamics. Treatment of voiding dysfunction may result in resolution of the reflux.
Although some children with lower grades of reflux may not require antibiotics, traditionally any child with reflux was maintained on prophylactic antibiotics to attempt to decrease the incidence of urinary tract infections. Several recent studies suggest that the practice of daily antibiotic prophylaxis in all children with reflux may be of limited benefit in preventing urinary tract infection. Prompt treatment of pyelonephritis prevents renal scar formation. Factors causing secondary reflux—such as dysfunctional voiding or obstruction—should be corrected.
In many children, reflux resolves with time. Reflux is graded as seen on voiding cystourethrography as follows:
Grade I: Contrast enters ureter
Grade II: Contrast enters the renal collecting system
Grade III: Slight dilation of the calices or ureter
Grades IV and V: Progressively increased amounts of caliceal dilation and ureteral dilation or tortuosity
Reflux most likely to resolve is of lower grade or detected at a younger age. Over 70% of children with grades I, II, or unilateral grade III reflux will have resolution within 5 years. Resolution in children with grade V or bilateral grade IV reflux can be anticipated in less than 10% of cases. Other factors that appear to negatively affect the chance for reflux resolution include early reflux during bladder filling, presentation with a febrile urinary tract infection, renal scars, and voiding dysfunction.
In obstructive secondary reflux (eg, posterior urethral valves), release of obstruction may cure reflux. Occasionally, surgical reimplantation is still required. In neuropathic reflux, intermittent catheterization for control of infection may allow return of valvular competence. However, many cases require bladder augmentation for a noncompliant bladder and ureteral reimplantation. In reflux associated with ectopic orifices, duplication with ureterocele, and other congenital malformations, reimplantation is generally required.
The aim of surgery is to correct the reflux. This is accomplished by the creation of a longer submucosal tunnel for the ureter. With bladder filling and increased pressure, the ureter is compressed between the mucosa and underlying detrusor muscle. This flap valve prevents reflux of urine. The necessary length of the tunnel to stop reflux depends on the diameter of the ureter, with a 5:1 length-to-diameter ratio being ideal. One of three methods is used in most cases: (1) in suprahiatal repair (Politano-Leadbetter procedure), a new ureteral hiatus is developed about 2.5 cm above the original one, and the ureter—after passing through a submucosal tunnel—is sutured to the cut edge of the trigone at the level of the original orifice. (2) In the cross-trigonal repair (Cohen procedure), the original hiatus is maintained, and the ureter is advanced through a submucosal tunnel, extending across the trigone to the contralateral bladder wall. (3) A totally extravesical ureteral advancement procedure (extravesical ureteroplasty) achieves results similar to those achieved with the intravesical methods, with a shorter hospital stay and shorter convalescence.
Injections of subureteric bulking agents have also been used to increase submucosal support of the ureter. With proper placement beneath the ureteral orifice under endoscopic vision, these injections act to bolster the deficient antireflux mechanism. Concern regarding late sequelae of Teflon injections (eg, particle migration) has prevented use of this approach in the United States. Currently, hyaluronic acid/dextranomer (NASHA/Dx) gel (Deflux) is the only FDA-approved material for endoscopic injection to manage vesicoureteral reflux in children. Short-term success in stopping reflux with the injection techniques appears to be around 75% overall, with most success with grades I-III reflux. The long-term success rates still need to be evaluated, as many studies only have short term (ie, 3 month) follow up.
[PubMed: 20368325].
The long-term prognosis is excellent for patients with mild to moderate reflux successfully treated with antibiotic prophylaxis. There are few instances of recurrent infection or renal insufficiency. Patients with more significant reflux or persistent urinary tract infections may benefit from subureteric injection or surgical reimplantation; the success rate is approximately 95% with the open surgical technique (cessation of reflux, clearance of renal infection, and absence of obstruction). Unfortunately, for patients with advanced disease (irreversible ureteral decompensation and severe bilateral scars), the prognosis is less favorable. These patients account for a significant proportion of patients with end-stage renal disease who ultimately require chronic dialysis, renal transplantation, or both.
Anomalies of the bladder are infrequent and include the following: (1) agenesis, or complete absence, which results in a persistent cloaca; (2) bladder duplication, which may be complete, with separate ureteral openings drained by duplicated urethras, or incomplete, with a septum or hourglass deformity; and (3) urachal anomalies, which in the most severe forms appear as a patent opening at the umbilicus and are usually associated with some form of bladder outlet obstruction. In less severe forms, a urachal diverticulum may be present at the dome of the bladder or a urachal cyst along the course of the partially obliterated urachus. These latter conditions may cause abdominal pain and umbilical or bladder infection requiring surgical treatment. Occasionally, adenocarcinoma develops in a urachal remnant (see section on Tumors of the Bladder).
Failure of cloacal division results in a persistent cloaca. Incomplete division is more frequent (though still rare) and results in a rectovesical, rectourethral, or rectovestibular fistula (usually with imperforate anus or anal atresia).
Exstrophy of the bladder is the most severe bladder anomaly—the result of a complete ventral defect of the urogenital sinus and the overlying inferior abdominal wall musculature and integument. The lower central portion is devoid of skin and muscle. The anterior bladder wall is absent, and the posterior wall is contiguous with surrounding skin. Urine drains onto the abdominal wall, the rami of the pubic bones are widely separated, and the open pelvic ring may affect gait. In males, the penis is shortened and the urethra is epispadiac. The exposed bladder mucosa tends to be chronically inflamed.
Currently, the favored treatment is bladder salvage, which includes closure of the bladder in the newborn period. Urethral closure and penile reconstruction have also been advocated at the time of the initial bladder closure. Ureteral obstruction or vesicoureteral reflux may develop and require ureteral reimplantation. The closed bladder may have a small capacity, and incontinence is often a complication. Patients frequently require multiple operations, including bladder augmentation and bladder neck reconstruction. Good results have been observed in more than half of all patients treated, with preservation of renal function and continence.
Prune belly syndrome consists of a triad of abnormalities: deficient abdominal wall musculature, bilateral cryptorchidism, and variable amounts of dilation of the urogenital tract. The cause is not known. Almost all children with prune belly syndrome have reflux. The incidence of eventual renal failure is 25%-30%. Risk factors for renal failure include bilateral abnormal kidneys on ultrasound or renal scan, a serum creatinine that never falls below 0.7 mg/dL, and clinical pyelonephritis. These children are managed with prophylactic antibiotics and frequent urine cultures, followed by prompt treatment of any urinary tract infections. Abdominoplasty may be performed to help correct the abdominal wall defect.
Congenital neurovesical dysfunction frequently accompanies a posterior myelomeningocele or sacral agenesis, with associated spinal abnormalities. Both conditions may result in incontinence and recurrent urinary infection with late sequelae (ureteral reflux, pyelonephritis, and renal failure). These children require frequent evaluation of their kidneys and kidney function because high bladder storage pressures may harm the kidneys.
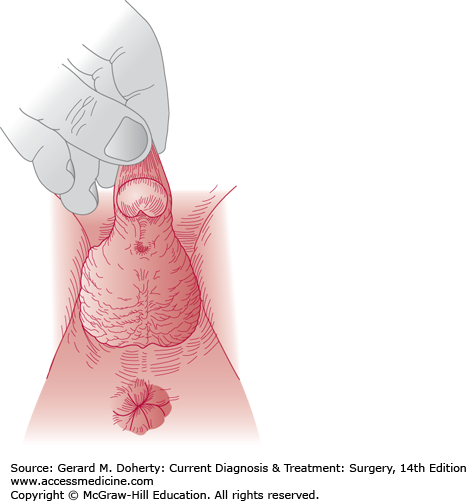
Hypospadias results from failure of fusion of the urethral folds on the undersurface of the genital tubercle. The urethral meatus is ventrally displaced on the glans on the shaft of the penis or more proximal at the level of the scrotum or perineum. With more proximal displacement, chordee (ventral curvature of the penile shaft) frequently occurs and requires treatment, or it precludes straight erections and normal intercourse (Figure 38–8). The midscrotal hypospadiac penis may resemble female external genitalia with an enlarged clitoris and labia. Sexual assignment in these latter infants requires hormonal and chromosomal analysis.
In hypospadias with the meatus positioned proximal to the corona, the prepuce is abnormal—not forming a complete cylinder due to a ventral defect. Circumcision should not be done in these patients, as the prepuce can be used later in surgical repair.
The degree of hypospadias dictates the need for repair. If the opening is glandular or coronal (85% of patients), the penis is usually functional both for micturition and procreation, and repair is done primarily for cosmetic reasons. Openings that are more proximal on the shaft require correction to allow voiding while standing, normal erection, and proper sperm deposition during intercourse. Surgical plastic repair of hypospadias is currently accomplished by a variety of highly successful one-stage operations and is routinely performed between 6 and 18 months of age. The most common complications of hypospadias surgery include meatal stenosis and fistula formation; however, improved techniques have decreased the incidence of these complications.
Epispadias is a rare congenital anomaly that is commonly associated with bladder exstrophy. When it occurs alone, it is considered a milder degree of the exstrophy complex.
The urethra opens on the dorsum of the penis, with deficient corpus spongiosum and loosely attached corpora cavernosa. If the defect is extensive, it may extend to the bladder neck, causing incontinence because of deficient sphincter muscles. The pubic bones are separated, as in exstrophy. Marked dorsiflexion of the penis is usually present.
Treatment consists of correction of penile curvature, reconstruction of the urethra, and reconstruction of the bladder neck in incontinent patients.
Congenital urethral strictures are rare but when present are most common in the fossa navicularis (just proximal to the meatus) and in the bulbomembranous urethra. Commonly, these strictures are thin diaphragms that may respond to simple dilation or to direct vision internal urethrotomy. Rarely is open surgical repair necessary. Congenital urethral strictures in girls and meatal stenosis in boys are uncommon. When the latter does occur, it appears to be acquired, as it is seen only in circumcised boys.
In males, urethral diverticula are nearly always in the pendulous or bulbous urethra. They are often associated with an obstructive flap of the urethral mucosa (anterior urethral valve), thought to represent incomplete closure of the urethral folds. Treatment by endoscopic unroofing is usually successful, though most diverticula are small and require no therapy. In females, they occur in adult life and are usually manifested by irritative symptoms and recurrent infection. The cause is unknown, but the disorder is most likely congenital. Treatment is usually by transvaginal excision. Diverticula may occasionally harbor stones or tumors.
Posterior urethral valves are the most common obstructive urethral lesion in newborn and infant males and the most common cause of end-stage renal disease in boys. They consist of obstructive folds of mucosa, which originate at or are attached at some point to the verumontanum in the prostatic urethra. The embryologic derivation is indefinite. They are partially obstructive and thus lead to variable degrees of back-pressure damage to the urinary bladder and upper urinary tract. Dilation and obstruction of the prostatic urethra are always present. Spontaneous urinary ascites from the kidneys is often seen in neonates. This clears when the obstruction is relieved.
About one-third of children with posterior urethral valves are now diagnosed by prenatal ultrasound. Another one-third are diagnosed in the first year of life, with the remaining third presenting later. Clinical manifestations consist of difficult voiding, a weak urinary stream, and a midline lower abdominal mass that represents a distended bladder. In some cases, the kidneys are palpable and the child may have signs and symptoms of uremia and acidosis. Urinary incontinence and urinary tract infection may occur. Laboratory findings include elevated serum urea nitrogen and creatinine and evidence of urinary infection. Ultrasound shows evidence of bladder thickening and trabeculation, hydroureter, and hydronephrosis. Demonstration of urethral valves on a voiding cystourethrogram establishes the diagnosis, as does endoscopic identification of valves. Up to 70% of children with valves may have vesicoureteral reflux.
Treatment consists of destruction of the valves by endoscopic incision. In a premature infant with a small urethra prohibiting transurethral resection, a temporary cutaneous vesicostomy may be required to provide drainage and improve impaired kidney function.
The prognosis depends on the original degree of kidney damage and the success of efforts to prevent or treat infection. Rates of chronic renal failure or end-stage renal disease range from 25% to 67% of boys with valves. Poor prognostic factors include the presence of bilateral reflux or an elevated nadir serum creatinine in the first year of life. Many of these children have delayed development of urinary continence due to bladder changes and impaired urinary concentration.
Neonatal testicular torsion (extravaginal torsion) is an extremely rare condition. The entire testicle and the tunica vaginalis are twisted. No trigger mechanism associated with the torsion has been identified. Although the vast majority are necrotic and nonsalvageable, several studies have reported salvage of testicular tissue when torsion is detected immediately following birth. Any scrotal swelling in the neonate requires close follow-up. Intravaginal testicular torsion in adolescents is described later in this chapter.
Congenital scrotal lesions include hypoplasia of the scrotum (unilateral or bilateral) in association with cryptorchidism and bifid scrotum with extensive hypospadias. Midline inclusion cysts may also occur.
True undescended testicles stop along the normal path of descent into the scrotum. They may remain in the abdominal cavity (least common), in the inguinal canal (canalicular), or just outside the external ring (suprascrotal, most common). Testes may also pass through the external ring and then be located ectopically, most commonly in a superficial inguinal pouch. The incidence of undescended testicles increases from 3% to 5% in full-term infants to 30% in premature infants. Most undescended testicles descend within the first 6 months of life, and by 1 year of age the prevalence is 1%. The left testicle is affected more often, and 1%-2% of children with cryptorchidism will have both testicles affected. Twenty percent of boys who present with cryptorchidism have one nonpalpable testis. Of nonpalpable testes, 20% are intra-abdominal; 40% are canalicular, scrotal, or ectopic testes; and 40% are atrophic or absent.
The diagnosis of cryptorchidism relies on physical examination. Absence of an identifiable testicle with ultrasound, computed tomography, or magnetic resonance imaging (MRI) does not prove testicular agenesis and therefore does not alter the need for surgical exploration. Testicular examination in the infant and young child requires two hands, with the first hand being swept from the anterior iliac spine along the inguinal canal to gently express any retained testicular tissue into the scrotum, which is palpated with the other hand. A true undescended or ectopic inguinal testis may slip or “pop” under the examiner’s fingers. To distinguish a retractile testicle, the testicle is brought into the scrotal position, holding it in place for a minute to fatigue the cremaster muscle. After this, a retractile testicle remains in the scrotum, whereas an ectopic or undescended testis immediately snaps back out of the scrotum. If a testis cannot be palpated in the inguinal canal or the scrotum, or in the typical ectopic sites, evaluation for a nonpalpable testis must be performed.
A child with bilateral nonpalpable testes should undergo hormonal evaluation for testicular absence. Elevations in luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and absence of detectable müllerian-inhibiting substance suggest testicular absence. Testicular absence is confirmed by a negative human chorionic gonadotropin (hCG) stimulation test. The hCG stimulation test is performed by the administration of intramuscular hCG (2000 IU/day for 3-4 days). Raised gonadotropin levels (FSH and LH) and a lack of a testosterone rise from hCG indicate bilateral absent testes, and a formal surgical exploration is unnecessary. When one or both components are lacking or there is detectable müllerian-inhibiting substance, surgical exploration is warranted.
Treatment of the undescended testicle offers the possibility of improved fertility, correction of patent processus vaginalis, prevention of testis torsion, and improvement in body image. There is controversy whether orchidopexy decreases the risk of malignancy, but placement of an undescended testicle in the scrotum assists physical examination of the testis. Histologic changes related to infertility occur in the undescended testicle as young as 1 year of age, and spontaneous descent rarely occurs after 6 months of age, making this the optimal time for surgical correction.
Almost 90% of undescended testes have an associated patent processus vaginalis, which predisposes to formation of a hydrocele or hernia. Occult inguinal hernia in patients with untreated undescended testis can present at any time with the typical symptoms or complications, including incarceration.
Prior to any surgical intervention, the patient is reexamined while under anesthesia because on occasion a retractile testicle descends under anesthesia or a previously nonpalpable testicle becomes palpable. For a palpable testicle, an open inguinal approach is performed. For the nonpalpable testicle in a child, a laparoscopic approach is preferred, but an open inguinal approach may be performed.
Success rates following orchidopexy are 74% for the abdominal testis, 87% for canalicular, and 92% for those distal to the external ring. The most significant complication is testicular atrophy, which occurs in 1%-2% of cases of orchidopexy, while complete devascularization of the testis is rare. Paternity rates have been reported at 65%, 90%, and 93% in men with bilateral cryptorchidism, unilateral cryptorchidism, and normally descended testicles, respectively. If only one testis is undescended, the sperm count is subnormal in 25%-33% patients, and serum FSH concentration is slightly elevated. These abnormalities suggest that both testes are abnormal, perhaps congenitally, although only one fails to descend. If both testes are undescended, sperm count usually is severely subnormal, and serum testosterone may be reduced.
Hormonal therapy is an option in the treatment of cryptorchidism because the condition may be related to hypogonadotropic hypogonadism. hCG is the only hormone approved for use in the treatment of cryptorchidism in the United States. Side effects of hCG treatment include enlargement of the penis, growth of pubic hair, increased testicular size, and aggressive behavior during administration. The likelihood of success with hormonal therapy is greatest for the most distal undescended testes or for testes that have been previously descended. Some suggest that hormonal therapy is effective only for retractile and not truly undescended testes. Although hormonal therapy may not be effective in achieving testicular descent, it may improve fertility in cryptorchid boys.
ACQUIRED LESIONS OF THE GENITOURINARY TRACT
Obstruction is one of the most important abnormalities of the urinary tract, since it eventually leads to decompensation of the muscular conduits and reservoirs, back-pressure, and atrophy of renal parenchyma. It also invites infection and stone formation, which cause additional damage and can ultimately end in complete unilateral or bilateral destruction of the kidneys.
Both the level and the degree of obstruction are important to an understanding of the pathologic consequences. Any obstruction at or distal to the bladder neck may lead to back-pressure affecting both kidneys. Obstruction at or proximal to the ureteral orifice leads to unilateral damage unless the lesion involves both ureters simultaneously. Complete obstruction leads to rapid decompensation of the system proximal to the site of obstruction. Partial obstruction leads to gradual progressive muscular hypertrophy followed by dilation, decompensation, and hydronephrotic changes.
Acquired urinary tract obstruction may be due to inflammatory or traumatic urethral strictures, bladder outlet obstruction (benign prostatic hyperplasia or cancer of the prostate), vesical tumors, neuropathic bladder, extrinsic ureteral compression (tumor, retroperitoneal fibrosis, or enlarged lymph nodes), ureteral or pelvic stones, ureteral strictures, or ureteral or pelvic tumors.
Regardless of its cause, acquired obstruction leads to similar changes in the urinary tract, which vary depending on the severity and duration of obstruction.
Proximal to the obstruction, the urethra dilates and balloons. A urethral diverticulum may develop, and dilation and gaping of the prostatic urethra and ejaculatory ducts may occur.
Early detrusor and trigonal thickening and hypertrophy compensate for the outlet obstruction, allowing complete bladder emptying. This change leads to progressive development of bladder trabeculation, cellules, saccules, and, finally, diverticula. Subsequently, bladder decompensation occurs and is characterized by the above changes plus incomplete bladder emptying (ie, postvoid residual urine). Trigonal hypertrophy leads to secondary ureteral obstruction owing to increased resistance to flow through the intravesical ureter. With detrusor decompensation and residual urine accumulation, there is stretching of the hypertrophied trigone, which appreciably increases ureteral obstruction. This is the mechanism of back-pressure on the kidney in the presence of vesical outlet obstruction (while the ureterovesical junction maintains its competence). Catheter drainage of the bladder relieves trigonal stretch and improves drainage from the upper tract.
A very late change with persistent obstruction (more frequently encountered with neuropathic dysfunction) is decompensation of the ureterovesical junction, leading to reflux. Reflux aggravates the back-pressure effect on the upper tract by transmitting abnormally high intravesical pressures and favors the onset or persistence of urinary tract infection.
The first change noted is a gradual increase in ureteral distention. This increases ureteral caliber and stimulates hyperactive ureteral contraction and ureteral muscular hypertrophy. Because the ureteral musculature runs in an irregular helical pattern, stretching of its muscular elements leads to lengthening as well as widening, causing the dilated ureter to assume a tortuous, serpiginous course, weaving back and forth across the relatively straight course of the ureteral vessels, which are unaffected by the ureteral obstruction. This is the start of ureteral decompensation, where tortuosity and dilation become apparent. These changes progress until the ureter becomes atonic, with infrequent, ineffective, or completely absent peristalsis.
The renal pelvis and calices, subjected to increased volumes of retained urine, distend. The pelvis shows evidence first of hyperactivity and hypertrophy and then of progressive dilation and atony. The calices show similar changes to a variable degree, depending on whether the renal pelvis is intrarenal or extrarenal. In the latter, caliceal dilation may be minimal in spite of marked pelvic dilation. In the intrarenal pelvis, caliceal dilation and renal parenchymal damage are maximal. The successive phases seen with obstruction are rounding of the fornices, followed by flattening of the papillae and finally clubbing of the minor calices.
With continued pelvicaliceal distention, there is parenchymal compression against the renal capsule and, more importantly, compression of the arcuate vessels results in a marked drop in renal blood flow leading to parenchymal ischemic atrophy. With increased intrapelvic pressure, there is progressive dilation of the collecting and distal tubules, with compression and atrophy of tubular cells.
The findings vary according to the site of obstruction.
Infravesical obstruction—Infravesical obstruction (eg, due to urethral stricture, benign prostatic hypertrophy, bladder neck contracture) leads to difficulty in initiation of voiding, a weak stream, and a diminished flow rate with terminal dribbling. Burning and frequency are common associated symptoms. A distended or thickened bladder wall may be palpable. Urethral induration due to stricture, benign prostatic hypertrophy, or cancer of the prostate may be noted on rectal examination. Meatal stenosis and impacted urethral stones are readily diagnosed by physical examination.
Supravesical obstruction—Renal pain or renal colic and gastrointestinal symptoms are commonly associated. Supravesical obstruction (eg, due to ureteral stone, UPJO) may be completely asymptomatic when it develops gradually over a period of months. An enlarged kidney may be palpable. Costovertebral angle tenderness may be present.
Evidence of urinary tract infection, hematuria, or crystalluria may be seen. Impaired renal function may be noted in cases of bilateral obstruction. Postrenal azotemia (serum changes reflecting impaired renal function due primarily to obstruction) is suggested by elevation of serum urea nitrogen and serum creatinine with a ratio greater than 10:1.
Radiologic examination is usually diagnostic in cases of stasis, tumors, and strictures. Dilation and anatomic changes occur above the level of obstruction, whereas distal to the obstruction, the configuration is usually normal. This helps in localizing the site of obstruction. Combined antegrade imaging by intravenous urograms and retrograde imaging by ureterograms or urethrograms is sometimes needed to demonstrate the obstructed segment. In supravesical obstruction, demonstration of stasis and delayed drainage is essential to establish and quantitate the severity of obstruction.
Ultrasonography—Ultrasonography reveals the degree of dilation of the renal pelvis and calices and allows for diagnosis of hydronephrosis even in the prenatal period. Color Doppler ultrasound can reveal blood flow and restrictive indices to help determine functional impairment.
Isotope studies—A technetium-99m DTPA scan or MAG-3 scan portray the degree of hydronephrosis as well as renal function. Use of diuretics during the scan can provide specific data on the significance of the obstruction and the need for treatment. Multiple studies can reveal ongoing functional changes.
CT scan—CT scan is of particular value in revealing the degree and site of obstruction as well as the cause in many cases. The use of contrast (CT urogram) agents allows estimation of residual renal function.
MR urogram—MRI provides anatomic images and identification of the site of obstruction. With dynamic contrast-enhanced MR urography, functional information is also obtained without the use of ionizing radiation.
Antegrade urography—Antegrade urography via percutaneous needle or tube nephrostomy is valuable when the obstructed kidney fails to excrete the radiopaque material on excretory urography. The Whitaker test requires percutaneous catheter access to the collecting system above the site of suspected obstruction. This permits fluid introduction into the renal pelvis and simultaneous measurement of urine flow rate and pressures in the bladder and renal pelvis, thus providing a quantitative assessment of the degree and severity of obstruction. The fluid transport can be measured and the degree of obstruction estimated by the use of a pressure monitor.
The most important complication of urinary tract obstruction is renal parenchymal atrophy as a result of back-pressure. Obstruction also predisposes to infection and stone formation, and infection occurring with obstruction leads to rapid kidney destruction.
The first goal of therapy is relief of the obstruction (eg, catheterization for relief of acute urinary retention). Definitive therapy often requires surgery, but minimally invasive techniques are becoming utilized more often. Simple urethral stricture may be managed by dilation or internal urethrotomy (incision of the stricture under direct vision through the resectoscope). However, urethroplasty (open surgical graft or flap of skin or buccal mucosa to replace urethral diameter) may be required and have better long-term success. Benign prostatic hyperplasia classically requires excision, but laser techniques are providing satisfactory outcomes with less morbidity. Impacted ureteral stones may either be removed or bypassed by a catheter unless it is thought that they may pass spontaneously.
Ureteral or UPJO requires surgical repair; however, endoscopic approaches within the ureter or by laparoscopy may be equal to open repair. Renal stones may be removed instrumentally via retrograde or antegrade percutaneous approach by direct extraction with baskets or by ultrasonic or laser lithotripsy or by irrigation through a tube placed directly into the kidney.
Preliminary drainage above the obstruction is sometimes needed to improve kidney function. Occasionally, intestinal urinary diversion or permanent nephrostomy is required. If damage is advanced, nephrectomy may be indicated.
The prognosis depends on the cause, site, duration, and degree of kidney damage and renal decompensation. In general, relief of obstruction leads to improvement in kidney function except in seriously damaged kidneys, especially those destroyed by inflammatory scarring.
Stenosis of the renal pelvis outlet is commonly due to congenital narrowing of the junction or compression by anomalous vessels. However, the lesion may be acquired. Presentation in adults often includes the abrupt onset of flank pain usually following ingestion of large amounts of fluids. Presentation in childhood is now most often made following the diagnosis of hydronephrosis by prenatal ultrasonography.
The diagnosis may be confirmed with a diuretic nuclear renal scan or intravenous urography, which reveals hydronephrosis with a dilated renal pelvis and slow drainage of either radiotracer or contrast medium. Occasionally, patients present with intermittent hydronephrosis and normal urograms, except during attacks of pain, when x-rays show typical obstruction. These patients generally have normal renal parenchyma. Retrograde ureteropyelography is usually needed in patients with chronic moderate to severe obstruction to determine the extent of the lesion and to provide assurance that the distal ureter is normal. Marked obstruction may make it difficult to determine whether kidney function is surgically salvageable. In these cases, it may be necessary to perform either (1) differential radioisotope renography with use of a diuretic during the study or (2) percutaneous nephrostomy and creatinine clearance by 24-hour urine collection.
Severe obstruction with minimal remaining renal function is best treated by unilateral nephrectomy. If renal function is adequate (> 10%-15% of total renal function or > 10 mL/min creatinine clearance), surgical repair of the stenosis, either by creation of a renal pelvis flap or by resection of the stenotic area and reanastomosis (dismembered repair), is warranted. Laparoscopic and/or robotic pyeloplasty has emerged as the standard of care in adults for the repair of UPJO. The use of ureteroscopy or percutaneous nephroscopy with endopyelotomy, incising the strictured ureteropelvic junction, offers an alternative method of therapy and is most useful in secondary UPJO, after primary repair has failed. Endoscopic approaches are less successful in the presence of a crossing vessel, poor renal function, and significant hydronephrosis. The surgical results of all the above methods are excellent in terms of functional preservation, improvement of urine flow, and relief of symptoms, but dilation of the calices may persist.
Ureteral stenosis can be secondary to congenital or acquired lesions. Congenital causes can include compression by an anomalous vessel such as a lower pole renal artery in UPJO or a retroperitoneal vein or primary megaureter where the distal ureter is partially obstructed. More commonly, the ureter is secondarily obstructed due to acquired conditions such as inflammation from chronic ureteral stones, trauma secondary to gynecologic or vascular surgery, or external penetrating trauma from a knife or gunshot wound. Enlarged pelvic lymph nodes or an iliac artery aneurysm or retroperitoneal fibrosis may obstruct the ureter, as can intrinsic ureteral cancer or bladder cancer infiltrating the ureter at its insertion into the bladder. Finally, infection such as urinary tuberculosis can cause distal ureteral strictures, and bilateral ureteral obstruction can occur from bladder neck obstruction with urinary retention secondary to benign prostatic hyperplasia.
Chronic conditions with slow development may not cause symptoms, whereas acute obstruction such as that from a stone will cause severe flank pain that may radiate to the groin or testes/labia. Diagnosis is most often made by a CT urogram with contrast that will show delayed function and a dilated renal pelvis and ureter down to the site of the obstruction. This is often an unsuspected finding on a CT done for other reasons in an asymptomatic patient.
Treatment depends entirely on the cause. Severe stenosis may require resection of the lesion and spatulated end-to-end anastomosis of the ureter. Less-severe obstruction may be managed by cystoscopy and ureteral or balloon dilation of the narrowed area under direct vision via a ureteroscope. Placement of an indwelling ureteral stent may dilate the stenosis over time and be a useful treatment as well in selected patients.
See also Chapter 22.
One or both ureters may be compressed by a chronic inflammatory process, usually of unknown cause, which involves the retroperitoneal tissues of the lumbosacral area. When the source of fibrosis is unknown, the entity is known as idiopathic retroperitoneal fibrosis. Patients treated for migraine with methysergide may develop this fibrosis. Sclerosing Hodgkin disease and fibrosis from metastatic cancer have also been implicated. Symptoms include flank pain, lower back and abdominal pain (from ureteral obstruction), and those associated with uremia. Some patients present with complete anuria. Urinary infection is unusual. If both ureters are obstructed, the serum creatinine is elevated, but unilateral renal obstruction from fibrosis may present with normal or slightly elevated creatinine levels.
Excretory urograms show hydronephrosis and a dilated ureter down to the point of obstruction. The ureters are displaced medially in the lumbar area. Retrograde ureterograms show a long segment of ureteral stenosis, though a catheter usually passes easily through the ureter. Ultrasound lacks the anatomic specificity to make the diagnosis, but obstruction proximal to the fibrotic mass yields hydronephrosis and hydroureter. Large areas of fibrosis can be identified with ultrasound. CT scans and MRI offer the most diagnostic information about the fibotic mass and both the degree and level of obstruction. If the patient has significant worsening of renal function, indwelling ureteral stent(s) or percutaneous nephrostomy tube(s) should be placed. When the patient’s condition has improved, definitive therapy can be accomplished. If methysergide is suspected to be the causative agent, fibrosis may subside when the drug is discontinued. When malignancy is in the differential diagnosis, a percutaneous or laparoscopic biopsy should be considered prior to treatment. These patients may benefit from administration of corticosteroids and/or other immunosuppressive agents. Chronic indwelling ureteral stents have also been used successfully. If these methods fail, ureterolysis must be performed to free the ureter from the fibrous plaque. The involved ureter should be dissected from the plaque, moved to a lateral position, and wrapped with omentum to prevent recurrent entrapment. This has been accomplished quite successfully with laparoscopic and robotic approaches.
The cause of benign prostatic enlargement is not known but is probably related to hormonal factors. The mechanism for opening and funneling the vesical neck at the time of voiding is altered by hyperplasia of the prostate, which causes increased outflow resistance. Consequently, a higher intravesical pressure is required to accomplish voiding, causing hypertrophy of the vesical and trigonal muscles. This may lead to the development of bladder diverticula—outpocketings of vesical mucosa between the detrusor muscle bundles. Hypertrophy of the trigone causes excessive stress on the intravesical ureter, producing functional obstruction and resulting in hydroureteronephrosis in late cases. Stagnation of urine can lead to infection; the onset of cystitis exacerbates the obstructive symptoms. The periurethral and subtrigonal prostate enlargement produces the most significant obstruction.
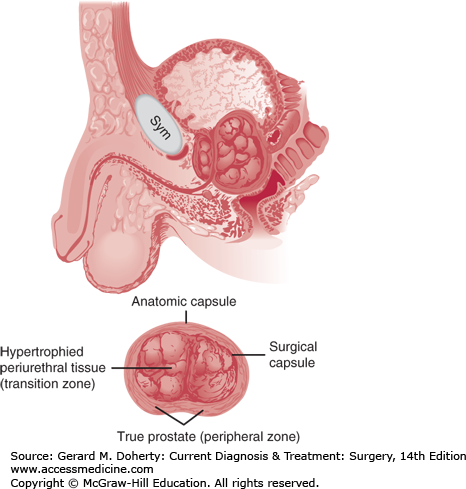
The prostate in young men has an anatomic capsule like an apple peel. In men with prostatic enlargement, there is a thick “surgical” capsule similar to an orange peel, composed of peripherally compressed true prostatic tissue (“peripheral zone”). The hyperplastic benign periurethral glands correspond to the “transition zone” and are the cause of the obstruction (Figure 38–9).
Typically, the patient has lower urinary tract symptoms and notices hesitancy and loss of force and caliber of the stream. The urgent need to void when the bladder is nearly full may be an early sign. He may also be awakened by the urge to void several times at night (nocturia). Postvoid dribbling (“terminal dribbling”) is particularly disturbing. The complication of infection increases the degree of obstructive symptoms and is often associated with burning on urination. Acute urinary retention may supervene. This is associated with severe urgency, suprapubic pain, and a distended, palpable bladder.
The size of the prostate rectally is not of primary diagnostic importance, since there is a poor correlation between the size of the gland and the degree of symptoms and amount of residual urine. The American Urological Association (AUA) developed a 7-item, self-administered questionnaire (AUA symptom score) that can assist the patient and physician in evaluating the patient’s lower urinary tract symptoms.
Urinalysis may reveal evidence of infection. Residual urine is commonly increased (> 50 cc), and a timed urinary flow rate is decreased (< 10-15 cc/s). The serum creatinine may be elevated in cases with prolonged severe obstruction.
Pelvic ultrasound can easily identify post-void urine residual as well as estimate prostate size and anatomy, in particular, the presence or absence of median lobe tissue. Bladder wall thickness, trabeculation, and diverticula can also be identified with ultrasound. Renal ultrasound can identify hydronephrosis in advanced cases of BPH. Transrectal ultrasound of the prostate provides better imaging of the prostate than the transabdominal approach but is more invasive and typically not required. Computerized tomography (CT), MRI, and excretory urography also provide anatomical information for an enlarged prostate, but are more time consuming and costly and should only be utilized in unusual circumstances (eg, ruling out malignancy).
Cystourethroscopy reveals secondary vesical changes (eg, trabeculation and diverticula) and enlargement of the prostatic urethra. Lateral lobe hypertrophy (so-called “kissing lobes”) as well as median lobe tissue can be easily visualized. Although cystoscopy is not required to make the diagnosis of BPH, it may identify other conditions in the differential diagnosis (eg, urethral stricture, bladder neck contracture, malignancy) as well as complications secondary to BPH (eg, bladder stones).
Simultaneous physiologic monitoring of bladder filling and emptying, urethral sphincter activity, abdominal pressure, and pelvic floor muscle activity (electromyography) can be extremely useful in documenting whether bladder outlet obstruction, poor bladder function, or other causes are responsible for lower urinary tract symptoms. While urodynamic studies are not required for diagnosis in all cases, they are helpful in cases with large postvoid residual volumes or underlying neurologic disease to help determine appropriate management.
Neurogenic bladder may produce a similar syndrome. A history suggesting a neuropathic etiology, such as diabetes mellitus, stroke, Parkinson’s disease, or spinal cord injury or compression, may be obtained. Neurologic deficit involving the spinal nerves S2-4 is particularly significant.
Cancer of the prostate also causes symptoms of vesical neck obstruction. Serum prostate-specific antigen (PSA) may be elevated in patients with benign prostatic hypertrophy, and the level increases as the volume of the prostate increases. Thus, an absolute value is not diagnostic, but in general, if it is over 10 ng/mL, the possibility of cancer should be evaluated.
Acute prostatitis may cause symptoms of obstruction, but the patient is septic and has infected urine. The prostate is exquisitely tender.
Urethral stricture diminishes the caliber of the urinary stream. There is usually a history of gonorrhea or local trauma. A retrograde urethrogram or cystoscopy shows the stenotic area. A stricture blocks the passage of an instrument or catheter.
Obstruction and residual urine lead to vesical and prostatic infection and occasionally pyelonephritis; these may be difficult to eradicate. Long-term obstruction can lead to renal failure.
The obstruction may lead to the development of bladder diverticula. Infected residual urine may contribute to the formation of calculi.
Functional obstruction of the intravesical ureter, caused by the hypertrophic trigone, may lead to hydroureteronephrosis.
The indications for operative management are impairment of or threat to renal function and bothersome symptoms. Because the degree of obstruction progresses slowly in most patients, conservative treatment may be adequate. Drugs that relax the prostatic capsule and internal sphincter (α-adrenergic blocking agents) or decrease the volume of the prostate (5α-reductase inhibitors or antiandrogens) have been tried with considerable success.
Treatment of chronic prostatitis may reduce symptoms. The resolution of a complicating cystitis usually affords some relief. In order to protect vesical tone, the patient should be cautioned to void as soon as the urge develops. Forcing fluids over a short time causes rapid vesical filling and decreasing vesical tone; this is a common cause of sudden acute urinary retention and thus should be avoided. Patients with urinary obstructive symptoms should avoid the use of cold remedies, including antihistamines and alpha agonists, because they are also a common cause of urinary retention. These conservative measures are of only temporary help—if any—in patients with BPH. There has been recent great interest, particularly by patients, in the use of phytotherapy for treatment of lower urinary tract symptoms, including saw palmetto, pumpkin seeds, and other plant extracts. Despite previous claims of efficacy based on small retrospective studies, a recent large, multicenter randomized trial of 369 men comparing placebo versus saw palmetto showed no improvement in AUA symptom score or secondary outcomes (eg, peak flow and PVR) in the saw palmetto group. Thus, the use of saw palmetto extracts for the treatment of BPH is not recommended.
[JAMA and JAMA Network Journals Full Text]
Controversy surrounds choices in the treatment of benign prostatic hyperplasia. No treatment (watchful waiting) may be appropriate in patients who complain of mild to moderate symptoms and thus have low AUA symptom scores and residual urine less than 70-100 cc. Interest has also focused on nonoperative medical therapy for those with more significant symptoms. α-Adrenergic blocking agents relax the internal (bladder neck) sphincter and prostatic capsule. Selective agents that are long-acting and preferentially work for this purpose include tamsulosin and silodosin. 5α-Reductase inhibitors block conversion of testosterone to DHT (the androgen active in promoting prostate growth) and are useful for large glands, particularly in combination with an alpha-blocker, which has been shown to best prevent urinary retention and other common progressive symptoms of prostatic obstruction. More recently, daily tadalafil, a PDE-5 inhibitor commonly used in the treatment of erectile dysfunction, has been shown to have efficacy in treating symptoms related to BPH.
Catheterization is mandatory for acute urinary retention. Spontaneous voiding may return, but a catheter should be left indwelling for at least a few days and preferably one week while detrusor tone returns. Several studies have documented improved success of voiding trials after retention if α-blockers are initiated for at least 48 hours prior to catheter removal. If several voiding trials fail, additional treatment is indicated.
Surgery for BPH is offered to men in whom medical therapy fails to provide adequate symptom relief and/or objective parameters such as post-void residual fail to improve. Additionally, men may request surgery because of poor compliance with medical therapy or desire to stop medications. Absolute indications for BPH surgery include recurrent infections, development of bladder stones, renal failure due to BPH, and persistent urinary retention due to obstruction. Men with large amounts of median lobe tissue (intravesical protrusion) tend to have a poor response to medical therapy and benefit most from early surgery.
There are two common approaches used in surgery for BPH: transurethral and transabdominal (open vs. minimally invasive). The transurethral route is by far the most common and preferred in patients with glands weighing less than 100 g because morbidity rates are lower and the hospital stay is shorter. With new technology, even larger glands can be managed with the transurethral approach. Larger glands, however, may require open surgery (suprapubic or retropubic enucleation), depending on the preference and experience of the urologist. Open surgery for BPH is the exception rather than the rule because of increased morbidity.
Transurethral approaches are the least invasive and require the shortest hospital stay (typically ambulatory or one night). The gold standard is the monopolar transurethral resection of the prostate (TURP), by which a resectoscope with a monopolar loop electrode is utilized to remove the obstructing BPH tissue. Hypotonic irrigation solution is required and hyponatremia due to excess systemic absorption (“TURP Syndrome”) is a unique complication of this approach. Additional complications of TURP include hematuria, UTI, stress incontinence, impotence, retrograde ejaculation, urethral stricture, and bladder neck contracture. Incontinence and impotence are rare complications in the hands of experienced surgeons (< 5%). TURP has reliably demonstrated improvement in AUA symptom score, flow rate, and PVR in randomized controlled trials. The more recent use of bipolar energy has allowed for the use of saline irrigation during TURP, eliminating the risk of hyponatremia.
Transurethral laser vaporization of the prostate with holmium or KTP (potassium titanyl phosphate) laser technology has demonstrated similar efficacy to TURP in well-selected patients with smaller glands. The major advantage of the laser approaches is less risk of bleeding, and even the ability to perform the procedure while patients remain on anticoagulation. Holmium-laser enucleation of the prostate (HOLEP) utilizes laser energy to enucleate the prostate adenoma (rather than vaporize the tissue). The tissue must then be morecellated to be removed via the resectoscope. HOLEP has similar efficacy to TURP and laser vaporization but may be more technically challenging.
An alternative transurethral approach for the treatment of BPH is transurethral incision of the prostate (TUIP). This procedure consists of a linear incision starting at the bladder neck and ending distally at the verumontanum, allowing expansion of the entire prostatic urethra. Typically two incisions are made with a cutting current at the 5 and 7 O’clock positions. TUIP is typically reserved for men with a steep/high bladder neck or small, obstructive prostates of 30 g or less in size.
Open and robotic enucleation of the prostate for BPH is reserved for larger glands where transurethral approaches would be unable to remove sufficient tissue to provide long-term relief of the obstruction.
Most patients with marked symptoms receive considerable relief and substantial improvement in urine flow following surgical treatment; however, those with milder forms may benefit from drug therapy. Unless absolute indications for surgery exist (see above), most men begin with medical therapy and progress to surgical interventions if substantial symptoms persist.
Acquired urethral strictures in men may be due to external trauma or to prior instrumentation (most common). Strictures may be inflammatory, due to gonorrhea, tuberculous urethritis, or schistosomiasis, or may rarely be a complication of cancer. The common presenting symptoms are dysuria, weak stream, splaying of the urinary stream, urinary retention, and urinary tract infection. Evidence of scarring due to trauma or induration and perineal fistula may be seen. Urethroscopy reveals the degree of narrowing. A retrograde urethrogram delineates the site and degree of stricture.
Urethral stricture must be differentiated from bladder outlet obstruction due to BPH, impacted urethral stones, urethral foreign bodies, and tumors.
Initial treatment consists of transurethral direct-vision internal urethrotomy (incision of the stricture). Successful results are obtained in 75% of patients. For long, dense strictures or those failing to respond to an initial internal urethrotomy, open surgical repair is indicated. This is probably best achieved by the transpubic or perineal route if the lesion involves the membranous urethra. If the bulbar urethra is involved, the perineal approach is indicated; if the distal/penile urethra is involved, the ventral penile approach is appropriate. End-to-end anastomosis is satisfactory for short segment strictures, usually 2 cm or less, with a more than 90% success rate. Longer strictures often require pedicle flaps for distal urethral strictures or onlay grafts (eg, buccal mucosa) for proximal strictures.
Hematuria, gross or microscopic, is a common urologic consult because it can be a presenting sign for underlying urologic malignancies. Red-colored urine may not necessarily include blood, and microscopic examination looking for red blood cells is prudent. Microscopic hematuria is defined as three or more red blood cells per high-powered field on urine microscopy in two of three properly collected specimens. The degree of hematuria bears no relation to the seriousness of the underlying cause. Urine dipstick test is the simplest method to check for blood and has a sensitivity of 91%-100% and a specificity of 65%-99%. Caution should be taken because false positives (menstrual blood, myoglobin, and hemolysis, among others) and false negatives (dipstick exposed to moisture and presence of reducing agents like ascorbic acid) can lead to confusing results. For this reason, further evaluation of microscopic hematuria performed only when microscopic analysis confirms the diagnosis. Knowledge of the medical history and medications can help rule out other causes of colored urine. Beets, rifampin, and phenazopyridine, among other substances, can cause urine discoloration. Anticoagulation at normal therapeutic levels does not predispose to hematuria, and patients should be evaluated for the hematuria, because 13%-45% of these patients may have significant urologic disease.
Ideally, a clean-catch midstream sample should be collected. If that is not possible, a catheterized specimen is indicated. It is important to note that hematuria can be due to urologic or renal parenchymal disease. The differential diagnosis thus includes benign causes like renal or bladder stones, papillary necrosis, urinary tract infections, prostatitis, or instrumentation, and malignant causes like renal, renal pelvis, bladder, prostate, or urethral cancer. Renal parenchymal causes of hematuria include glomerular and interstitial renal disease. These patients may have proteinuria and casts on the urinalysis, and the red blood cells are typically dysmorphic. Nephrology evaluation is required for hematuria associated with glomerular or interstitial renal disease.
Patients with hematuria referred to urology are classified into low-risk and high-risk groups. High-risk groups include smokers, age older than 40 years, history of exposure to pelvic radiation or cyclophosphamide, occupational exposure to chemicals or dyes, and history of urinary tract infections or other urological disorders. It is recommended that a complete workup be performed for all patients with symptomatic hematuria, all patients with gross hematuria, and high-risk patients with microscopic hematuria. The workup includes history and physical examination, serum creatinine, upper tract imaging (typically CT urogram), cystoscopy, and urine cytology. Asymptomatic patients younger than 40 years who have microscopic hematuria and have no risk factors can be evaluated with upper tract imaging and either cystoscopy or voided cytology, as the risk of significant pathology in this population is very low. If the workup is negative, it is recommended that the patient be evaluated with urinalysis, voided cytology, and blood pressure check at 6, 12, 24, and 36 months.
URINARY TRACT INFECTIONS
Urinary tract infection is the second-most common type of infection in humans and is frequently encountered by primary care physicians as well as urologists.
These infections are caused by a variety of pyogenic bacteria that typically produce a nonspecific tissue response. The most common organisms are gram-negative bacteria, particularly Escherichia coli. Less common are Enterobacter aerogenes, Proteus vulgaris, Proteus mirabilis, Pseudomonas aeruginosa, and Enterococcus faecalis.
Owing to the short length of the female urethra and bacterial colonization of the introitus, ascending infection is a common occurrence in young girls and in sexually active women. In men, ascending infection is often a consequence of urethral instrumentation.
Although relatively uncommon, descending or hematogenous urinary tract infection is usually associated with local urinary tract disorders—most commonly, obstruction and stasis; less commonly, trauma, foreign bodies, or tumors.
Lymphatic spread occasionally occurs from the large bowel or from the cervix and adnexa in the female through the perivesical and periureteral lymphatics.
Direct extension to the urinary bladder of nearby inflammatory processes (eg, appendiceal abscess, enterovesical fistula, or pelvic abscess) may occur.
Infection is usually initiated or sustained by predisposing factors. Predisposing systemic factors include diabetes mellitus, immunosuppression, and malnutrition; these disorders likely interfere with normal bladder and body defense mechanisms. Predisposing local factors include incontinence, constipation, organic or functional obstruction, stasis (residual urine), foreign bodies (especially catheters and stones), tumors, or necrotic tissue. Vesicoureteral reflux facilitates transport of bacteria from the bladder to the kidney, which subsequently predisposes to pyelonephritis.
Urinary tract infection is classified as (1) upper urinary tract infection (most commonly acute or chronic pyelonephritis or infection due to renal abscess), (2) lower urinary tract infection (cystitis or urethritis), or (3) genital infection (prostatitis, epididymitis, seminal vesiculitis, or orchitis).
In the absence of urinary tract infection, surgery of the upper urinary tract should require only short-term prophylactic antibacterial therapy. In the presence of infection, one attempts to sterilize the system before operation. If stenting or tube drainage is required and there are no symptoms of infection, colonization does not call for antibacterial therapy until the stent or tube is to be changed or removed. Broad-spectrum antibacterial prophylaxis is started at that time.
With lower urinary tract surgery, antibacterial therapy is advised before operations involving the urethra and the bladder, especially for women in whom contamination from vaginal organisms is likely. Men undergoing prostatectomy for obstructive prostatism often have urinary tract infection, particularly when catheter drainage is used preoperatively. In these cases, antimicrobial therapy is necessary before and after surgery to prevent bacteremia.
In the presence of urinary tract infection, any urethral instrumentation poses a threat of bacteremia, and possibly sepsis—more apt to occur in men than in women. Appropriate antibacterial coverage should be instituted before manipulation.
After a short-term single catheterization, the rate of infection is 1%-5%. However, in certain patients—pregnant women, elderly, or debilitated patients—and in the presence of urologic disease, the risk is much higher. An indwelling catheter often leads to bacterial colonization, especially in women. The incidence is proportionate to the duration of catheterization and reaches approximately 95% after 5 days.
Strict aseptic technique is of critical importance in catheterization. Proper cleansing of the genitalia is essential. Iodophor preparations may be used for cleaning the vaginal introitus or the glans penis. Many common urinary tract pathogens are present in normal colonic flora, and these organisms often gain access to the urinary tract of catheterized patients. Cross-contamination of urinary catheters (passive transmission of bacteria from patient to patient on the hands of hospital personnel) is a frequent mode of transfer of resistant organisms. Measures directed to the prevention of catheter cross-contamination are essential. Closed catheter drainage is probably the best way to reduce cross-contamination.
With sterile technique during catheterization and a closed drainage system, most catheters can be kept sterile for 48-72 hours. In a closed drainage system, an added airlock or one-way valve preventing reflux of urine from the collecting bag to the draining tubes also helps prevent infection. The general principles are as follows: (1) Indwelling catheters should be used only when absolutely necessary. (2) Catheters should be inserted with strict aseptic technique. (3) A closed drainage system, preferably with a one-way valve, is advisable. (4) Nonobstructed dependent drainage is essential. (5) Unnecessary irrigation of the system should be avoided. (6) If the catheter is needed for a prolonged period, it should be changed every 2-4 weeks to minimize encrustation and stone formation. (7) Catheterized patients with asymptomatic catheter colonization should be given antibiotics just before the catheter is changed or removed—not during the period of catheterization unless symptomatic infection occurs.
Imaging of the urinary tract is recommended in every febrile infant or young child following the first urinary tract infection. Imaging includes a renal and bladder ultrasound and a voiding cystourethrogram. The renal ultrasound may detect hydronephrosis, duplication anomalies, stones, or abnormalities of the bladder wall and should be obtained at the earliest convenient time. A cystogram may be obtained by instillation of contrast medium with fluoroscopy or by instillation of a radionuclide. Radionuclide cystography has the advantage of decreased radiation, while the contrast-voiding cystourethrogram has the advantage of providing better anatomic detail, which may help detect bladder or urethral abnormalities. Either method should include a voiding phase because reflux is the most likely abnormality to be detected and may only occur with voiding. The cystogram should be obtained once the child is free of infection.
Imaging recommendations in adults with a urinary tract infection vary depending on the patient’s past history and present symptoms. Renal/bladder ultrasound is a good initial study to identify upper- (eg, hydronephrosis, stones, or abscess) and lower- (eg, urinary retention or bladder calculi) tract sources of infection. CT scan with intravenous contrast can identify stigmata of pyelonephritis (see below).
The choice of antibiotics depends on the type of organism and its sensitivity, as determined by urine cultures. For uncomplicated infection, adequate urine concentrations of the antibiotic determine efficacy, but in cases of bacteremia and septic shock, serum concentrations are crucial. Commonly used oral medications are sulfonamides, nitrofurantoin, ampicillin, trimethoprim-sulfamethoxazole, fluoroquinolones, and oxytetracycline. For parenteral therapy, aminoglycosides and cephalosporins are effective against the most common organisms (ie, P. mirabilis, E. aerogenes, and P. aeruginosa).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree