INTRODUCTION
Plastic surgery, although considered a technique-oriented and multiregional specialty, is in essence a problem-solving field. The training of a plastic surgeon allows him or her to see surgical problems in a different light and select from a variety of options to solve these surgical problems. Plastic surgeons have received broad training, and many have completed residencies in other fields such as general surgery, otolaryngology, orthopedics, urology, or neurosurgery. Other modalities of training have more recently integrated these and other surgical subspecialties into a more comprehensive training program.
The basic principles of plastic surgery are careful analysis of the surgical problem, careful planning of procedures, precise technique, and atraumatic handling of tissues. Alteration, coverage, and transfer of skin and associated tissues are the most common procedures performed. Plastic surgery may deal with the closure of surgical wounds—particularly recalcitrant wounds such as those occurring postradiation or poorly healing wounds in immunocompromised patients. Plastic surgery also deals with the removal of skin tumors, repair of soft tissue injuries including burns, correction of acquired or congenital deformities, or enhancement of undesirable cosmetic features. Craniofacial and hand surgery, also within the realm of plastic surgery, may require additional surgical training.
In the past quarter century, increased knowledge of anatomy and the development of many new techniques have brought about important changes in plastic surgery. It is now known that in many areas the blood supply of the skin is derived principally from vessels arising from underlying muscles and larger perforating blood vessels rather than solely from vessels of the subcutaneous tissue, as was formerly thought. One-stage transfer of large areas of skin, fascia, and muscle tissue can be accomplished if the axial pedicle of the underlying fascia or muscle is included in the transfer. With the use of microsurgical techniques, musculocutaneous units or combinations of bone, fascia, muscle, and skin can be successfully transferred and vessels and nerves less than 1 mm in size can be repaired. These so-called free-flap transplantations are a major advance in the treatment of defects that were previously untreatable or required lengthy or multistaged procedures. More sophisticated knowledge of the blood supply to the skin has introduced the concept of perforator flaps whereby one perforating vessel is identified that may supply a large segment of overlying skin and subcutaneous tissue. Similarly, the concept of neurocutaneous flaps has given rise to the design of additional flap territories such as the sural flap in the lower leg and the sensate radial flap in the forearm.
The plastic surgeon, as a member of the craniofacial surgical team, is able to dramatically improve the appearance and function of children with severe congenital deformities. Children of normal intelligence who previously had been social outcasts are now able to lead relatively normal lives. Improved understanding of facial growth and abnormal development and diagnostic techniques such as the CT scan, MRI, and 3D computer-assisted imaging enable the reconstructive surgeon to develop a complex strategy for remodeling the deformed craniofacial skeleton. This may involve remodeling or repositioning of part or all of the cranial vault, the orbits, the midface, and the mandible. These complex and at times formidable reconstructions are performed by moving specific skeletal units and adding autogenous bone grafts. These structures are kept in place using miniplate fixation; the miniplates are made of titanium or resorbable material.
A notable advance in craniofacial surgery was the introduction of distraction osteogenesis, which borrows from the Ilizarov principle of distraction. A cortical cut is made in the bone, and a distraction apparatus is applied so that in measured amounts (usually 1 mm per day) the bone is either stretched to offset a length discrepancy or transported to bridge a gap. In craniofacial surgery, it is more commonly brought to bear to enlarge or cause overgrowth of areas such as an underdeveloped mandible.
Additional areas of involvement for the plastic surgeon entail allotransplantation, particularly with the increasing number of clinical limb allotransplants, which unfortunately still require immunosuppression. It is hoped that immunotolerance will someday become a reality, allowing transplantation of nonessential organs with a minimum of dangerous immunosuppression. Transplantation of the hand with excellent functional recovery in some cases has been performed successfully but still requires a great deal of immunosuppression. Face transplants have been performed with some initial success. The first facial transplant was performed in France and consisted of a partial segment of the face. The functional recovery to date has been remarkable. The problems of facial animation still need to be refined. Additionally, a number of ethical issues with regard to facial identity and immunosuppression require further resolution.
Tissue engineering of bone, cartilage, and nerve is an area of ongoing research for plastic surgeons. Although encouraging experimental results have been reported in anatomic areas difficult to reconstruct, such as the external ear, the nose, or the larynx, there are as yet few clinical applications.
Fetal surgery for cleft disorders and scar considerations, an area pioneered by a number of plastic surgeons, appears to be in a quiescent stage, particularly because the persistent real and potential risks to the fetus and mother may not be warranted for disorders that are not life threatening. Significant technical advances in the postnatal treatment of cleft lip and cleft palate have also lessened the enthusiasm for fetal surgery for these disorders.
I. GRAFTS & FLAPS
A graft of skin detaches epidermis and varying amounts of dermis from its blood supply in the donor area and is placed in a new bed of blood supply from the base of the wound, or recipient area. The way a skin graft survives or “takes” is first by diffusion of nutrient elements from the graft bed, known as imbibition; then after a period of 2-5 days, the graft actually revascularizes from the bed, a process known as inosculation. Although the technique is relatively simple to perform and generally reliable, definite considerations about the donor area and adequacy of the recipient area are important. Skin grafting is a quick, effective way to cover a wound if vascularity is adequate, infection is not present, and hemostasis is assured. Color match, contour, durability of the graft, and donor morbidity must be considered.
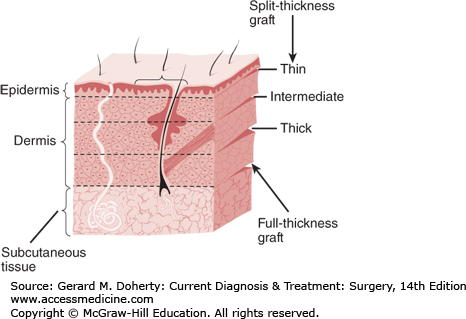
Skin grafts can be either split-thickness or full-thickness grafts (Figure 41–1). Each type has advantages and disadvantages and is indicated or contraindicated for different kinds of wounds (Table 41–1).
| Type of Graft | Advantages | Disadvantages |
|---|---|---|
| Thin split-thickness | Survive transplantation most easily. Donor sites heal most rapidly. | Fewest qualities of normal skin. Maximum contraction. Least resistance to trauma. Sensation poor. Aesthetically poor. |
| Thick split-thickness | More qualities of normal skin. Less contraction. More resistant to trauma. Sensation fair. Aesthetically more acceptable. | Survive transplantation less well. Donor site heals slowly. |
| Full thickness | Nearly all qualities of normal skin. Minimal contraction. Very resistant to trauma. Sensation good. Aesthetically good. | Survive transplantation least well. Donor site must be closed surgically. Donor sites are limited. |
Thinner split-thickness grafts (0.01-0.015 inch) become vascularized more rapidly and survive transplantation more reliably. This is important in grafting on less than ideal recipient sites, such as contaminated wounds, burn surfaces, and poorly vascularized surfaces (eg, irradiated sites). A second advantage is that donor sites heal more rapidly and can be reused within a relatively short time (7-10 days) in critical cases such as major burns.
In general, however, the disadvantages of thin split-thickness grafts outweigh the advantages. Thin grafts exhibit the highest degree of postgraft contraction, offer the least amount of resistance to surface trauma, and are least like normal skin in texture, suppleness, pore pattern, hair growth, and other characteristics. Hence, they are usually aesthetically unacceptable.
Thicker split-thickness skin grafts (> 0.015 inch) contract less, are more resistant to surface trauma, and are more similar to normal skin than are thin split-thickness grafts. They are also aesthetically more acceptable but not as acceptable as full-thickness grafts.
The disadvantages of thick split-thickness grafts are relatively few but can be significant. They are less easily vascularized than thin grafts and thus result in fewer successful takes when used on less than ideal surfaces. Their donor sites are slower to heal (requiring 10-18 days) and heal with more scarring than donor sites for thin split-thickness grafts—a factor that may prevent reuse of the area.
Meshed grafts are usually thin or intermediate split-thickness grafts that have been rolled under a special cutting machine to create a mesh pattern. Although grafts with these perforations can be expanded from 1.5 to 9 times their original size, expansion to 1.5 times the unmeshed size is the most useful. Meshed grafts are advantageous because they can be placed on an irregular, possibly contaminated wound bed and will usually take. Also, complications of hemostasis are fewer because blood and serum exude through the mesh pattern. The disadvantage is poor appearance following healing (alligator hide look).
Donor sites for split-thickness grafts heal spontaneously by epithelialization. During this process, epithelial cells from the sweat glands, sebaceous glands, or hair follicles proliferate upward and spread across the wound surface. If these three structures are not present, epithelialization will not occur.
Full-thickness skin grafts include the epidermis and all the dermis. They are the most aesthetically desirable of the free grafts because they include the highest number of skin appendage elements, undergo the least amount of contracture, and have a greater ability to withstand trauma. There are several limiting factors in the use of full-thickness grafts. Since no epidermal elements remain to produce epithelialization in the donor site, it must be closed primarily, and a scar will result. The size and number of available donor sites is therefore limited. Furthermore, conditions at the recipient site must be optimal in order for transplantation to be successful.
Areas of thin skin are the best donor sites for full-thickness grafts (eg, the eyelids and the skin of the postauricular, supraclavicular, antecubital, inguinal, and genital areas). Submammary and subgluteal skin is thicker but allows camouflage of donor area scars. In grafts thicker than approximately 0.015 inch, the results of transplantation are less reliable, except on the face, where vascularity is usually superior.
A composite graft is also a free graft that must reestablish its blood supply in the recipient area. It consists of a unit with several tissue planes that may include skin, subcutaneous tissue, cartilage, or other tissue. Dermal fat grafts, hair transplant grafts, and skin and cartilage grafts from the ear fall into this category. Obviously, composite grafts must be small or at least relatively thin and will require recipient sites with excellent vascularity. These grafts are generally used in the face.
Epithelial cells, grown or cultured in a special medium in vitro, will coalesce into thin sheets that can be used to cover full-thickness wounds. Although these cultured epithelial sheets were first used in the treatment of burns, the result was somewhat unsatisfactory because the coverage was very fragile and disfiguring. More recently, success has been obtained with artificial dermis, which when placed in an appropriate bed will revascularize and can then be covered by a very thin (0.05 cm) split-thickness skin graft, cultured or otherwise. This artificial dermis is increasingly being used in the treatment of burns. Modifications of this concept have also been applied to the care of chronic ulcers, particularly in the leg. The artificial dermis is made out of a collagen matrix and has very low or no antigenicity.
Research continues to develop bioengineered products that are becoming more common place in plastic surgery and general wound management. The source of these products varies (eg, human, porcine, etc) and can alter the products indications and overall ability to assist with wound management. Some of the more common products used are Integra and acellular dermal matrices (ADM), Integra is a bilayer material consisting of interwoven bovine collagen and a glycosaminoglycan that mimics the dermal layer of skin. This has made it ideal for reconstruction cases where more than simple skin grafts will suffice, such as over a tendon. ADM is acellular cadaver skin that is chemically prepared. Although initially designed for burn reconstruction, it has been used in wound care and general reconstruction cases from head to toe.
Instruments used for obtaining skin grafts include razor blades, skin grafting knives (Blair, Ferris Smith, Humby, Goulian), manual drum dermatomes (Padgett, Reese), and electric or air-powered dermatomes (Brown, Padgett, Hall, Zimmer). The electric and air-powered dermatomes are the most widely used because of their reliability and ease of operation. A surgeon, even with only limited experience, can successfully obtain sheets of split-thickness skin grafts using the electric dermatomes.
To ensure survival of the graft, there must be (1) adequate vascularity of the recipient bed, (2) complete contact between the graft and the bed, (3) adequate immobilization of the graft-bed unit, and (4) relatively few bacteria in the recipient area.
Because survival of the graft is dependent upon growth of capillary buds into the raw undersurface of the graft, vascularity of the recipient area is of prime importance. Avascular surfaces that will not generally accept free grafts are tissues with severe radiation damage, chronically scarred ulcer beds, bone or cartilage denuded of periosteum or perichondrium, and tendon or nerve without their paratenon or perineurium, respectively. For these surfaces, a bed capable of producing capillary buds must be provided; in some cases, excision of the deficient bed down to healthy tissue is possible. All unhealthy granulation tissue must be removed, because bacterial counts in granulation tissue are often very high. If bone is exposed, it can be decorticated down to healthy cancellous bone with the use of a chisel or power-driven burr, and a meshed split-thickness skin graft can be applied. If an adequate vascular bed cannot be provided or if the presence of essential structures such as tendons or nerves precludes further debridement, skin or muscle flaps are generally indicated for coverage.
Inadequate contact between the graft and the recipient bed can be caused by collection of blood, serum, or lymph fluid in the bed; formation of pus between the graft and the bed; or movement of the graft on the bed. The use of fibrin sealants (Artiss) has more recently been developed to assist with skin graft take. This is sprayed over the wound bed prior to placement of any skin graft. After 60-90 seconds, a fibrin clot develops that better holds the skin graft in place and diminishes potential spaces between the wound bed and the skin graft, which can negatively affect skin graft survival.
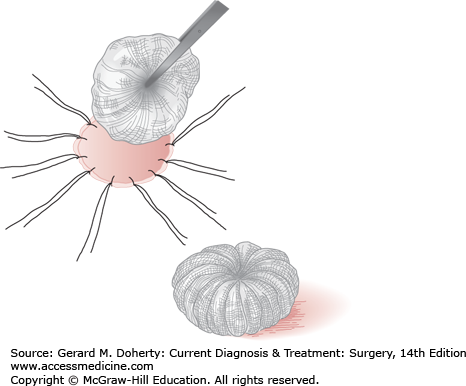
After the graft has been applied directly to the prepared recipient surface, it may or may not be sutured in place and may or may not be dressed. Whenever the maximum aesthetic result is desired, the graft should be cut exactly to fit the recipient area and precisely sutured into position without any overlapping of edges. Very large or thick split-thickness grafts and full-thickness grafts will usually not survive without a pressure dressing. In areas such as the forehead, scalp, and extremities, adequate immobilization and pressure can be provided by circular dressings. Tie-over pressure stent dressings are advisable for areas of the face, where constant pressure cannot be provided by simple wraparound dressings; areas where movement cannot be avoided, such as the anterior neck, where swallowing causes constant motion; and areas of irregular contour, such as the axilla. The ends of the fixation sutures are left long and tied over a bolus of gauze fluffs, cotton, a sponge, or other suitable material (Figure 41–2).
Grafts applied to freshly prepared or relatively clean surfaces are generally sutured or stapled into place and dressed with pressure. The use of fibrin sealants has helped minimize the need for these usually uncomfortable bolster techniques. A single layer of damp or other nonadherent fine-mesh gauze is applied directly over the graft. Immediately over this are placed several thicknesses of flat gauze cut in the exact pattern of the graft. On top of these is placed a bulky dry dressing of gauze fluffs, cotton, a sponge, or other material. Pressure is then applied by wraparound dressings, adhesive tape, or a tie-over pressure stent dressing. An alternative dressing is to place a nonadherent fine-mesh gauze atop the graft followed by a negative-pressure dressing. The vacuum-assisted dressings may be useful for irregular contours, such as around digits and webspaces or joint surfaces, by maintaining wound-to-graft interface, immobilizing the grafted area, suctioning serosanguineous fluid, and possibly promoting neovascularization.
In many cases, it is permissible—and sometimes even preferable—to leave a skin graft site open with no dressing. This is particularly true in slightly infected wounds, where the grafts tend to float off in the purulent discharge produced by the wound. These wounds are best treated with meshed grafts so that liquid forming between the graft and the wound bed can exude and be removed without disturbing the graft. This treatment can also be used for noninfected wounds that produce an unusual amount of serous or lymphatic drainage, as occurs following radical groin dissections.
In severely ill patients, such as those with major burns, where time under anesthesia must be kept to a minimum, large sheets of meshed split-thickness skin grafts are rapidly applied but not sutured. Skin staples may be used to fix the graft rapidly. Grafts need not be dressed if the area is small, but if the area is large or circumferential, a dressing should be applied. Meshed grafts should generally be covered for 24-48 hours to prevent dryness, because their dermal barrier has been partly disrupted.
Skin graft dressings may be left undisturbed for 5-7 days after grafting if the grafted wound was free of infection, if complete hemostasis was obtained, if fluid collection is not expected, and if immobilization is adequate. If any one of these conditions is not met, the dressing should be changed within 24-48 hours and the graft inspected. If blood, serum, or purulent fluid collection is present, the collection should be evacuated—usually by making a small incision through the graft with a scalpel blade and applying pressure with cotton-tipped applicators. The pressure dressing is then reapplied and changed daily so that the graft can be examined and fluid expressed as it collects.
The ideal donor site would provide a graft identical to the skin surrounding the area to be grafted. Because skin varies greatly from one area to another as far as color, thickness, hair-bearing qualities, and texture are concerned, the ideal donor site (such as upper eyelid skin to replace skin loss from the opposite upper eyelid) is usually not found. However, there are definite principles that should be followed in choosing the donor area.
In general, the best possible color match is obtained when the donor area is located close to the recipient area. Color and texture match in facial grafts will be much better if the grafts are obtained from above the region of the clavicles. However, the amount of skin obtainable from the supraclavicular areas is limited. If larger grafts for the face are required, the immediate subclavicular regions of the thorax will provide a better color match than areas on the lower trunk or the buttocks and thighs. When these more distant regions are used, the grafts will usually be lighter in color than the facial skin in Caucasians. In people with dark skin, hyperpigmentation occurs, producing a graft that is much darker than the surrounding facial skin.
Donor sites of split-thickness grafts heal by epithelialization from the epithelial elements remaining in the donor bed. The ability of the donor area to heal and the speed with which it does depends on the number of these elements present. Donor areas for very thin grafts will heal in 7-10 days, whereas donor areas for intermediate-thickness grafts may require 10-18 days and those for thick grafts 18-21 days or longer.
Because there is a normal anatomic variation in the thickness of skin, donor sites for thicker grafts must be chosen with the potential for healing in mind and should be limited to regions on the body where the skin is thick. Infants, debilitated adults, and elderly people have thinner skin than healthy younger adults. Grafts that would be split-thickness in the normal adult may be full thickness in these patients, resulting in a donor site that has been deprived of the epithelial elements necessary for healing.
The donor site itself can be considered a clean open wound that will heal spontaneously. After initial hemostasis, the wound will continue to ooze serum for 1-4 days, depending on the thickness of the skin taken. The serum should be collected and the wound kept clean so that healing can proceed at a maximal rate. The wound should be cared for as described above for clean open wounds in either of two ways.
One technique of donor site management is an open (dry) technique. The donor site is dressed with porous, sterile fine-mesh or nonadherent gauze. After 24 hours, the dry gauze is changed but the nonadherent gauze is left on the wound and exposed to the air, a heat lamp, or a blow dryer. A scab will form on the gauze and will peel off from the edges as epithelialization is completed underneath. This method has the advantage of simple maintenance once the wound is dry.
The second method that has become more popular is the closed (moist) technique. Studies have demonstrated that the rate of epithelialization is enhanced in a moist environment. In contrast to the dry technique, pain can be reduced or virtually eliminated. Moist-to-moist gauze dressings that require frequent wetting have been replaced by newer synthetic materials. A gas-permeable membrane (OpSite, Tegaderm) that sticks to the surrounding skin provides an artificial blister over the wound. Occasionally, there is a break in the protective seal covering leakage of serum collected under the membrane. This increases the risk of infection, especially in a contaminated zone. Newer hygroscopic dressings actually absorb and retain many times their weight in water. They are permeable to oxygen yet impervious to bacteria. Infection is still a concern; however, because of occasional exposure of the wound during healing. Newer dressings, such as Mepilex, contain silver-impregnated ions that control bacterial contamination and may hasten healing and reepithelialization while keeping the patient more comfortable. Silver ion is exquisitely antimicrobial and is used for burn dressing care as well as skin graft sites.
The term “flap” refers to any tissue used for reconstruction or wound closure that retains part or all of its original blood supply after the tissue has been raised and moved to a new location. That part still connected through which the blood supply enters and exits is referred to as the flap base or pedicle. With local skin flaps, a section of skin and subcutaneous tissue is raised from one site and moved to a nearby area, with the base remaining attached at its original location.
Flaps can be classified according to the pattern of blood supply to the skin into random or axial pattern. Flaps can further be classified according to their tissue content into muscle, musculocutaneous, fasciocutaneous, and others.
Random pattern flaps consist of skin and subcutaneous tissue cut from any area of the body in any orientation, with no distinct pattern or particular relation to the blood supply of the skin of the flap. Such flaps receive their blood supply from vessels in the subdermal tissue. Although commonly used, this is the least reliable type of flap, and except when cut from facial and scalp skin, the ratio of length to width cannot safely exceed 1.5:1. Its use should be minimized. Presently, in any reconstructive effort, one should use a flap with known reliability and a predictable blood supply.
The axial pattern flap has a well-defined arteriovenous system running along its long axis. Because of good vascular supply, it can be made comparatively long in relation to width. Foremost among the axial flaps are the deltopectoral and the forehead flaps, which are based on perforating branches of the internal mammary artery and supraorbital and supratrochlear or superficial temporal vessels, respectively. Other axial flaps are the groin flap, based on the superficial circumflex iliac artery; the dorsalis pedis flap, based on the artery of the same name; the radial forearm flap; the scapular flap; the lateral upper arm flap; and various scalp and face flaps.
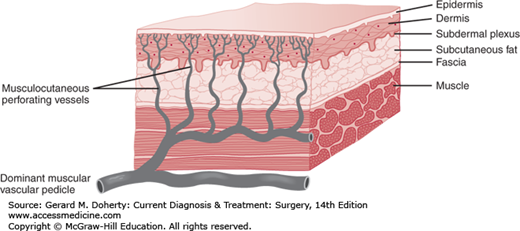
Musculocutaneous flaps consist of skin and underlying muscle, which provide reliable coverage with usually one operation. The use of musculocutaneous units has developed as surgeons have gained more knowledge of the way in which blood is supplied to the skin. The technique has revolutionized reconstructive surgery.
The subdermal plexus of vessels from which skin flaps derive their blood supply is augmented or directly supplied in many areas by sizable perforating vessels arising from underlying muscles. Many muscles receive their blood supply from a single axial vessel, with only minor contributions from other sources (Figure 41–3). The skin over these muscles can be completely circumscribed and elevated in continuity with the underlying muscle up to its major vascular pedicle. If the vessels in the pedicle are preserved, the unit can be moved in wide arcs to distant areas of the body while normal or near normal blood flow is continued to the skin island as well as to the muscle. The donor sites of such flaps can often be closed primarily.
Knowledge of the anatomy of muscles and their nerve and blood supply is necessary for the successful design of musculocutaneous flaps. Although almost any skeletal muscle can be used, muscles with a dominant arterial pedicle and reliable perforating vessels to the skin are most useful.
In addition to their reliability, musculocutaneous flaps clean up recipient sites that are heavily contaminated with bacteria better than skin flaps do. This is why muscle-containing flaps are the best choice for coverage of wounds caused by radiation or osteomyelitis or those that have a high probability of infection.
The most commonly used muscles and musculocutaneous flaps are the latissimus dorsi, pectoralis major, tensor fasciae latae, rectus femoris, rectus abdominis, trapezius, temporalis, serratus anterior, gluteus maximus, gracilis, and gastrocnemius muscles.
The latissimus dorsi musculocutaneous unit is supplied by the thoracodorsal vessels. Use of this unit has been widely applied in the one-stage reconstruction of the breast following radical or modified radical mastectomy (see section on Rectus Abdominis). The entire latissimus dorsi muscle can be detached from its origin and transposed to the anterior chest. An island of skin can also be included in the center of the muscle to restore the skin lost on the anterior chest wall. Refinements in technique utilize only enough muscle to carry the skin island, thus leaving intact a good portion of innervated, functional muscle. This unit is also useful for coverage of defects on the anterior chest, shoulder, head and neck, and axilla and even for restoration of flexion of the elbow. It is a popular muscle for free tissue transfer because of its long and relatively large and reliable vascular pedicle.
The pectoralis major musculocutaneous unit obtains its vascular supply from the thoracoacromial axis of the subclavian artery just medial to the medial border of the pectoralis minor. It derives a dual blood supply from medial intercostal perforators branching from the internal mammary artery. The entire unit may be transposed medially, especially after disinsertion from the humerus, to cover defects of the sternum, neck, and lower face. Also, an island of skin can be outlined low on the chest and made to reach intraoral defects following cancer excision.
The trapezius musculocutaneous unit, based on the descending branch of the transverse cervical artery, is useful for covering defects in the neck, face, and scalp. When skeletonized as an island, the flap will reach the top of the head. When it is used in conjunction with a neck dissection, the transverse cervical artery must be preserved. Functional preservation of shoulder elevation may be accomplished by selectively sparing the transverse, superior fibers of the muscle.
The temporalis muscle extends from the temporal fossa to the coronoid process of the mandible. It is supplied by the deep and superficial temporal systems. It is commonly used to fill orbital defects. However, it can cover neighboring cranial, maxillary, palatal, and pharyngeal regions.
The tensor fasciae latae musculofascial unit is supplied by the lateral femoral circumflex artery, a branch of the profunda femoris. It has a wide arc of rotation anteriorly and posteriorly. It is elevated with the fascia lata and thus can be used to reconstruct the lower abdominal wall. It has been used to cover defects following excision of osteoradionecrotic ulcers of the pubis or groin. It is also the method of choice for coverage of greater trochanteric pressure ulcers.
The rectus femoris, a more robust flap than the tensor fasciae latae with a shorter arc of rotation, has supplanted the latter for reconstruction of the lower abdominal wall and for coverage to postradiation ulcers in the pubis and groin. It has a dual blood supply: a muscular branch from the profunda femoris and an axial branch from the superficial femoral artery to the overlying skin and fascia.
The rectus abdominis is supplied by the deep superior and inferior epigastric vessels that run in the undersurface of the muscle and anastomose with the segmentally arranged intercostal vessels to form the epigastric arcade. These vessels send perforating branches throughout the length of the muscle, perforating the anterior rectus sheath and supplying the overlying skin. The transverse rectus abdominis myocutaneous (TRAM) flap, when based on the superior epigastric vessel and including the infraumbilical skin, has become a workhorse for autologous tissue breast reconstruction. In situations of marked deformity, such as a radical mastectomy associated with radiation therapy or previous abdominal surgery, reconstruction of the breast can be accomplished reliably with infraumbilical skin and adipose tissue based on both rectus muscles. This superiorly based TRAM flap involves an abdominoplasty as well as reconstruction of the breast. It is a technically demanding operation but gives a very satisfying result. When based on the deep inferior epigastric vessel and using the supraumbilical skin (the “flag” flap), the flap can cover defects of the abdominal wall, flank, groin, and thigh. Using the inferior epigastric vessels to transport the skin and adipose tissue by means of microvascular surgery (see section on Free Flaps) has become a popular method of breast reconstruction. A small portion of the rectus muscle or just a main perforator vessel that supplies the overlying fat and skin is taken. This flap is known as the deep inferior epigastric perforator flap, or DIEP flap (see section on Perforator Flaps).
The gluteus maximus is useful as a muscle or musculocutaneous unit for covering pressure sores or traumatic defects over the sacrum and ischium. The muscle has a double blood supply from the superior and inferior gluteal arteries to the respective halves of the muscle. In ambulatory patients, it is advisable to perform a function-preserving operation by advancing the muscle medially and preserving its insertion laterally.
The gracilis muscle receives its dominant blood supply proximally from the medial femoral circumflex artery. Its arc of rotation makes it an excellent source of coverage for ischial pressure sores and vaginal reconstruction. Other recent uses have included transportation of the muscle alone for repair of a persistent perineal sinus following abdominal-perineal resection.
The gastrocnemius musculocutaneous unit is based on either the medial or lateral head of the muscle. Each head is supplied by a sural artery, a branch of the popliteal artery that enters the muscle at its most proximal third near its origin. The flap is most useful to cover defects of the knee and proximal anterior tibia. Coverage of exposed bone in the middle and lower leg, where this unit cannot reach, can be accomplished by use of local muscle flaps such as the soleus. Complex bone and soft tissue injuries of the middle and lower leg may require reconstruction with free muscle flaps.
A plexus of vessels is located on top of the muscular fascia and is supplied from vessels that run within the intermuscular septa. These vessels tend to run axially along the fascia, sending perforators to the skin at intervals. Flaps can be designed that are safer than random flaps and that need not contain an entire muscle unit for their transfer. Furthermore, it is possible to make fasciocutaneous or septocutaneous flaps that safely exceed the traditional limits of a 1.5:1 ratio between length and width. Examples of fasciocutaneous flaps are those overlying the gastrocnemius, quadriceps, and rectus abdominis muscles. Other commonly used flaps are the radial forearm, lateral arm, scapular, and deltopectoral flaps.
Anatomic studies have confirmed the presence of an arterial pedicle accompanying a sensory nerve such as the sural nerve. Consequently, one may be able to outline a skin territory over the trajectory of a sensory nerve with good viability of the overlying skin.
Free flaps involve tissue transplantation using microvascular surgery. The term is actually incorrect, because the blood supply from the main axial pedicle of the flap is completely detached and then reattached at a distance to recipient vessels near the wound area.
An operating microscope with two viewing binocular lenses, specialized instruments, and swaged-on needles of 60-80 μm are required for microsurgery; 8-0, 9-0, and 10-0 suture is used to anastomose vessels as small as 0.5 mm in diameter.
Examples of free flaps in current use are axial pattern skin and fasciocutaneous flaps, such as scapular, groin, radial forearm, and anterolateral thigh, which are used when only skin and subcutaneous tissue are needed, and muscle and musculocutaneous flaps, such as latissimus dorsi, gracilis, and rectus abdominis flaps, which are used when the bulk and vascularity of muscle are needed. Composite free flaps such as the fibular flap with its overlying skin are most helpful free flaps for reconstruction of the mandible as well as the floor of the mouth following head and neck tumor extirpations.
The vascular pedicle areas of some flaps contain functional nerves, which can also be reattached with microscopic guidance. Examples are inferior gluteal, thigh, and tensor fasciae latae flaps, which contain sensory nerves. Attempts using sensory flaps to provide protective sensation in critical areas such as the feet or the ischium in paraplegic patients have so far been clinically unsuccessful. More encouraging is the work being done to provide sensibility to the floor of the mouth with a sensory innervated radial forearm flap. Motor flaps can restore functions such as forearm flexion or facial expression.
Bone and functional joints can be transplanted as free flaps. Flaps from the ribs, fibula, and iliac crest have all been successfully transferred to areas such as the mandible and tibia. The toe-to-thumb transfer is an example of a complex transplantation, which includes bone with a functional joint, tendons, and nerves as well as skin.
A sophisticated variation on the use of the musculocutaneous principle has been the development of perforator flaps. This usually entails taking a branch from the major vascular pedicle that may perforate the muscle to arborize and form a subcutaneous vascular plexus that will supply a considerable amount of overlying skin. Perhaps the greatest benefit from a perforator flap is decreased donor site morbidity. Structures such as the fascia, muscle, and associated nerves may be preserved while allowing the skin to be used for reconstruction.
The DIEP flap exemplifies this well for autologous tissue breast reconstruction. While maintaining the same skin territory as the TRAM flap, the perforating vessels are carefully dissected away from the rectus abdominis. By sparing the muscle, there is potentially a reduction in excessive abdominal wall weakness at the donor site.
The anterolateral thigh flap has become the mainstay for cutaneous flaps at some institutions. Based on musculocutaneous perforators from the vastus lateralis, it can be used when a relatively thin cutaneous flap is needed, such as in head and neck reconstruction. The donor site may be closed primarily depending on the flap width.
The perforator concept has been applied to further territories of skin over the perforator segments of the gluteal, thoracodorsal, and medial plantar arteries among others.
II. PRINCIPLES OF WOUND CARE
There are many types of wounds and many factors to consider when choice of coverage procedure is made. Skin type and color, glandular association, and hair-bearing characteristics must be considered. Avascular wound beds, such as exposed bone, cartilage, or tendon, will not accept skin grafts unless viable periosteum, perichondrium, or paratenon (respectively) is present. Other areas with poor vascularity are joint capsules, radiation-damaged tissue, and heavily scarred tissue. Exposed or implanted alloplastic material cannot be used as a graft bed. Such areas must be covered with tissue that is attached to its own blood supply. Skin flaps can be used but are sometimes inadequate because their blood supply is tenuous and the layer of subcutaneous fat is even less reliably vascular and may not attach to the underlying avascular surface. Muscle or musculocutaneous flaps are generally required for avascular areas.
The coverage tissue may need to have more bulk than the original tissue. Areas such as bony surfaces and prominences, weight-bearing surfaces, densely scarred areas, and areas of potential pressure breakdown may require thick, durable covering. Again, skin grafts or skin flaps may not be of adequate thickness even though they may survive and cover the wound. Musculocutaneous flaps are more successful. Bulkiness may be undesirable in areas such as the scalp, face, neck, or hand. Defects in these areas that for other reasons require a musculocutaneous flap for coverage may need to be debulked in a secondary procedure. Axial skin flaps or free axial pattern flaps may be a better choice than musculocutaneous flaps in some areas.
Contraction begins during the proliferative phase of healing and continues to a large degree in wounds covered only by split-thickness skin grafts. The grafted area may shrink to 50% of its original size, and both the graft and surrounding tissue may become distorted. Splinting of the area for 10 days or longer may favorably alter contraction. Full-thickness skin grafts rich in dermis, attached to a fresh wound bed, will considerably reduce contraction, and skin flaps will eliminate it altogether. In an orifice or tubular passageway, such as the nasal airway, pharynx, esophagus, or vagina, absence of contraction is critical.
The effects of atrophy and gravity should also be considered when technique of coverage is chosen. A denervated muscle will atrophy up to 60% of its regular size. The muscle tissue in a musculocutaneous flap will atrophy even when the nerve to the muscle is preserved in the pedicle, because the muscle’s functional tension is generally not restored. Gravity will cause sagging of any tissue that does not have enough plasticity or muscle dynamics to counteract gravitational pull. Reconstructions in the face often tend to sag.
Wounds at risk for or known to have bacterial contamination also require certain types of coverage (eg, pressure sores, lower extremity defects, and wounds resulting from incision and drainage of abscesses). If the area can be skin grafted, meshed split-thickness grafts are most effective, because bacterial exudate will not collect under these grafts. Musculocutaneous flaps are associated with fewer residual bacteria over time than are random pattern skin flaps. This is probably due to the vastly superior vascularity of musculocutaneous flaps.
Contaminated wounds or wounds that are exuding a considerable amount of fluid can be treated by negative-pressure or vacuum wound dressings. This entails the application of a sponge-like material connected to a suction device that keeps the wound dry as it suctions the excess exudates. The negative pressure on the wound also appears to have a positive effect on healing and increased revascularization. It has become a popular method of preparing a wound for definitive closure.
Wounds associated with nearby injuries that will probably require further surgery (eg, injuries to tendons or nerves) should be covered with flaps, because the flaps can be incised or undermined to allow for additional surgery. Skin grafts do not have sufficient vascularity to allow for these procedures.
The ideal type of wound closure is primary approximation of the skin and subcutaneous tissues immediately adjacent to the wound defect, producing a fine-line scar and the optimal aesthetic result in skin texture, thickness, and color match.
All excisions and wound closures should be planned with this ideal in mind. Obviously, large lesions cannot be excised and closed primarily. With invasive cancers, such as sarcomas, the primary goal is performance of adequate en bloc resection, with the type of wound closure being of secondary importance. Nevertheless, even larger excisions, such as mastectomies, can be planned with definite consideration for closure and subsequent reconstruction.
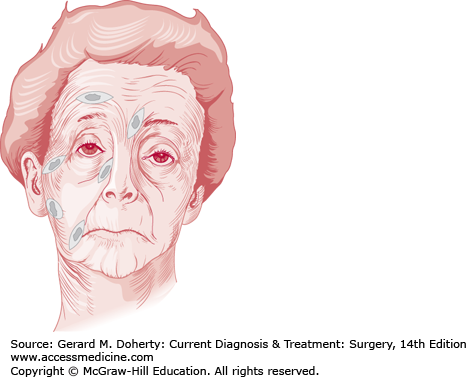
In most cases, minimal scars can be achieved only if the line or lines of incision are placed in, or parallel to, the skin lines of minimal tension. These lines lie perpendicular to the underlying muscles. On the face, they are obvious as wrinkles or lines of facial expression that become more pronounced with age, since they are secondary to repeated muscle contraction (Figure 41–4). On the neck, trunk, and extremities, the lines of minimal tension are most noticeable as horizontal lines of skin relaxation on the anterior and posterior aspects of areas of flexion and extension.
Langer lines, which were determined by cadaver study, probably show the direction of fibrous tissue bundles in the skin and are no longer considered accurate guides for placing skin incisions.
If the lines of expression cannot be followed, the line of incision should (if possible) be placed at the junction of unlike tissues such as the hairline of the scalp and the forehead, the eyebrow and the forehead, the mucosal and skin junction of the lips, or the areolar and skin margins of the breast. Scars will be partially hidden if incisions are placed in inconspicuous areas such as the crease of the nasal ala and cheek, the auricular-mastoid sulcus, or the submandibular-neck junction. Lines of incision should never purposely cross flexor surfaces such as the neck, axilla, antecubital fossa, or popliteal space or the palmar skin creases of the fingers and hand, because of the risk of contracture formation. A transverse oblique or S incision should be incorporated when crossing these sites.
If a lesion is to be excised, an elliptic incision placed parallel to the skin lines of minimal tension will give the best result if the amount of tissue to be excised does not preclude primary closure.
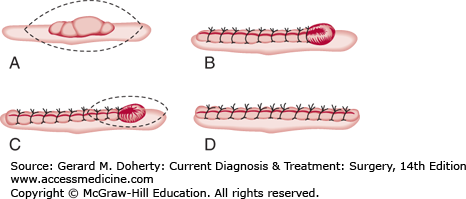
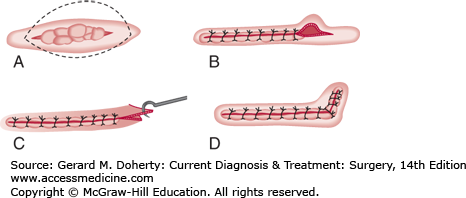
If the ellipse is too broad or short, a protrusion of skin, commonly called a “dog-ear,” will occur at each pole of the wound closure (Figure 41–5). This is most easily corrected by excising the dog-ear as a small ellipse.
A dog-ear may also be present if one side of the ellipse is longer than the other (Figure 41–6). In this case, it may be easier to excise a small triangle of skin and subcutaneous tissue from the longer side.
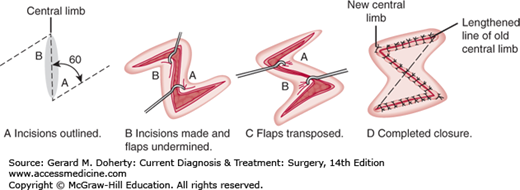
One of the most useful and commonly used techniques in primary wound closure is the Z-plasty. The procedure is illustrated in Figure 41–7. The angles formed by the Z-shaped incision are transposed as shown in order (1) to gain length in the direction of the central limb of the Z or (2) to change the line of direction of the central limb of the Z. Ninety-degree angles would provide the greatest gain in length of the central limb, but smaller angles, such as 60-degree angles, are usually used, because the incision is easier to close and significant gain in length is still achieved. The Z-plasty is used for scar revision and reorientation of small wound incisions so that the main incision will be in a more ideal location. The lengthening function is used for the release or breakup of scar contractures across flexion creases. Frequently, many small Z-plasties in series rather than one large one are done. Occasionally, incisions will be placed under excessive vertical tension after the release of an underlying contracture, such as Dupuytren contracture in the hand.
Suture technique in primary closure is important but will not compensate for poorly planned flaps, excessive tension across the incision, traumatized skin edges, bleeding, or other problems. Sometimes even a skillfully executed closure may result in an unsightly scar because of healing problems beyond the control of the surgeon.
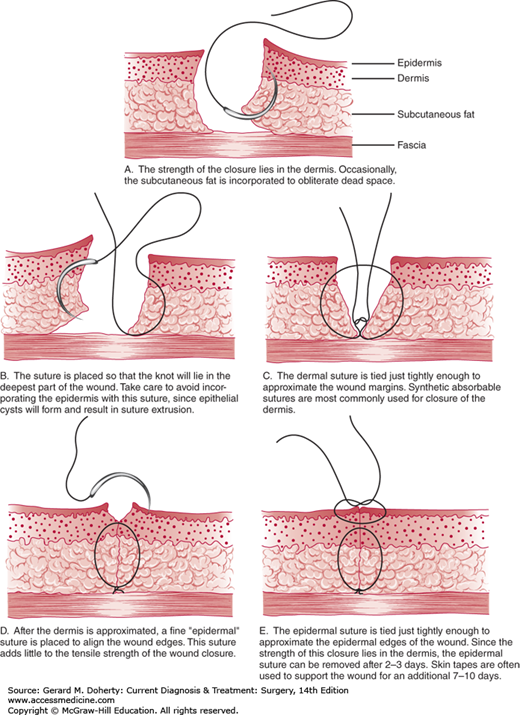
The goal of closure is level apposition of dermal and epithelial edges with minimal or no tension across the incision and no strangulation of tissue between sutures. This is usually accomplished by placement of a layer of interrupted or running absorbable sutures in the superficial fascia and subdermal level at the base of the dermis. This suture prevents tension from forming in the upper dermis and epithelium and also causes the surface planes to be level. The epithelial edges can then be opposed with interrupted or running monofilament sutures of absorbable or permanent material. The absorbable suture is placed in the subcuticular or intradermal plane and is left in place. The permanent sutures are removed quickly according to the region of the body (within 3-4 days in the face), so the suture tracks can be avoided. Sterile adhesive tape (Steri-Strips) placed across the incision will also prevent surface marks and can be used either primarily or after surface sutures have been removed. Taping will not correct errors in suturing that have resulted in uneven edges or tension across the incision. Tape burns may occur if there is excessive tension or swelling around the incision.
The size and even the type of suture material are less important than careful suture placement and observance of previously mentioned factors. Almost any suture properly placed and removed early enough will provide closure without leaving suture marks. The use of monofilament nylon or polypropylene suture material is advised, however, because these types of sutures cause the fewest reactions of currently available suture materials, excluding stainless steel. Running subcuticular, pullout-type monofilament sutures may be left in for up to 3 weeks without causing reactions. Even buried nylon sutures are well tolerated and generally cause fewer problems than braided or absorbable sutures.
An alternative to sutures is the use of skin adhesives such as 2-octylcyanoacrylate (Dermabond). It works well in small areas without much tension or shearing. It is also advisable in children. Further studies are needed to evaluate its wider applicability.
Table 41–2 shows some of the indications for choice of coverage in various types of wounds. Once a given type of flap is chosen, there are still at least two major considerations in the selection of the exact flap to be used. The most significant consideration is the degree of injury that will occur in the donor area. There is always a trade-off when tissue is taken from one area and used in another. This trade-off is minimal when a well-designed, well-placed skin flap leaves a donor defect that can be closed primarily, but the trade-off is great when the donor defect is as severe as the original wound (eg, skin graft donor sites that become infected or musculocutaneous donor sites that fail to heal).
| Type of Wound | Type of Coverage | Reason for Choice |
|---|---|---|
| Mildly (< 105) infected wounds (including burns) | Thin split-thickness or meshed | Difficulty in obtaining successful take of thicker grafts. Donor sites may be reused sooner. |
| Significantly (> 105) infected wounds (osteomyelitis) | Thin split-thickness or meshed skin grafts or muscle or musculocutaneous flaps | Rich muscle vascular supply can sterilize an infected wound. |
| Wounds with poorly vascularized surfaces | Thin split-thickness skin grafts or flaps | Difficulty in obtaining successful take of thicker grafts. Flap with intrinsic blood supply may be required. |
| Small facial defects | Full-thickness skin graft or local flap | Produces best aesthetic result. |
| Large facial defects | Thick split-thickness skin grafts or flaps | Cannot use full-thickness graft, because of limited size of donor sites. |
| Full-thickness eyelid loss | Local flap or composite graft | Repair requires more than one tissue element. |
| Deep loss of nasal tip | Local flap or composite graft | Repair requires thicker tissue than present in split- or full-thickness grafts. |
| Avulsive wounds with exposed tendons and nerves | Flap | Requires thick protective coverage without graft adherence to tendons and nerves. |
| Exposed cortical bone or cartilage | Skin or muscle flap | Free grafts will not survive on avascular recipient site. |
| Wounds resulting from radiation burns | Muscle or musculocutaneous flap | Free grafts will not survive on avascular recipient site. Damaged tissue extends deeper than may be apparent. |
The patient can often participate in the choice of donor locations and should certainly be made aware of potential donor site scars and complications. The tendency has been to use muscle flaps instead of musculocutaneous flaps to permit easy primary closure of the donor site. The muscle can then be resurfaced with a split-thickness skin graft during the same procedure to give a satisfactory result. This provides for an acceptable donor site scar rather than risking disruption of a tight closure or an otherwise ugly donor site.
The second consideration in selection of a flap is that some or all of the graft or flap may be lost. In general, if the patient’s overall condition is poor or the loss of a flap would result in a devastating defect, a very reliable type of flap should be chosen. For example, a microvascular anastomosis can be performed on a leg with one remaining arteriosclerotic vessel to the foot, but if the anastomosis fails, the vessel may thrombose and the leg may be lost. In this case, a flap that is safer, although more time consuming to place, may be chosen, such as a cross-leg flap.
Additional considerations in reconstructive surgery involve the technique of elevating and transposing flaps. For random skin flaps, these considerations include proper length-to-width ratio, careful planning to allow for transposition with minimal tension and adjustments at the recipient site, accurate dissection in the subcutaneous plane to avoid injury to the subdermal plexus, and avoidance of folding or kinking of the flap. Surgical technique must be atraumatic, and hemostasis must be achieved. With axial pattern flaps, the surgeon must have knowledge of the important underlying blood vessels as well.
Closure technique is as important as elevation and transposition technique. Flaps should not be allowed to dry out. The wound bed should be irrigated. Closed-system, nonreactive suction drains are routinely used in both the wound bed and the donor defect for most flaps of any significant size. Suction evacuates blood or serum that may accumulate and keeps the flap firmly pressed against the wound bed. External pressure is both ineffective and detrimental for these purposes. Sutures should accurately and completely appose skin edges without strangulating the epithelium, particularly on the flap side. Buried half-mattress (flap) sutures are recommended (Figure 41–8). Dressings over flaps should be minimal and should not cause pressure or constriction. Emollient dressings, such as petrolatum gauze, antibiotic ointment, or silver sulfadiazine cream, have been shown to aid in preventing desiccation and subsequent necrosis of areas of marginal vascularity.
After a flap is at least temporarily tacked into its final position, adequacy of vascularity can be determined by intravenous injection of fluorescein dye, 10-15 mg/kg, and examination under ultraviolet light (Wood light). Areas that fluoresce within 10 minutes following dye injection can be expected to survive. Areas that do not fluoresce usually lack arterial inflow, which may be due to temporary arterial spasm but is often due to insufficient perfusion that will result in necrosis. A good clinical evaluation of the flap on the operating table is usually sufficient. Any sign of mottling or cyanosis or flap congestion that indicates a degree of venous obstruction warrants serious consideration of reexploration.
III. SPECIFIC DISORDERS TREATED BY PLASTIC SURGERY
In response to any injury severe enough to break the continuity of the skin or produce necrosis, the skin heals with scar formation. Under ideal circumstances, a fine, flat hairline scar will result. The details of wound healing are presented in Chapter 6.
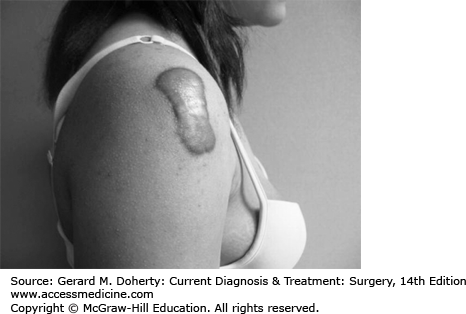
However, hypertrophy may occur, causing the scar to become raised and thickened, or a keloid may form. A keloid is a true tumor arising from the connective tissue elements of the dermis. By definition, keloids grow beyond the margins of the original injury or scar; in some instances, they may grow to enormous size. (Figure 41–9)
Hypertrophic scars and keloids are distinct entities, and the clinical course and prognosis are quite different in each case. The overreactive process that results in thickening of the hypertrophic scar ceases within a few weeks—before it extends beyond the limits of the original scar—and in most cases, some degree of maturation occurs and gradual improvement takes place. In the case of keloids, the overreactive proliferation of fibroblasts continues for weeks or months. By the time it ceases, an actual tumor is present that typically extends well beyond the limits of the original scar, involves the surrounding skin, and may become quite large. Maturation with spontaneous improvement does not usually occur.
More recent research has shown differences on a biochemical level that can be quite complex. In essence though, it is understood that fibroblasts in both disorders display an increase in procollagen. This is compensated for in hypertrophic scars but not in keloids. This results in an increased ratio of type 1 to type 3 collagen in keloids.
The most frustrating aspect of hypertrophic and keloid treatment for both patient and care provider alike is the high incidence of recurrence. Much work has gone into developing techniques to avoid and treat these scar problems, with mixed results. As this has become more evident, providers have become more specialized and aggressive with treatment options. More recent studies looking at treatments such as interferon, 5-flurouracil injections and bleomycin injections have shown some promise. But no therapy has shown to conclusively treat these problems.
Since nearly all hypertrophic scars undergo some degree of spontaneous improvement, they do not require treatment in the early phases. If the scar is still hypertrophic after 6 months, surgical excision and primary closure of the wound may be indicated, but recurrence is between 45% and 100% if no other treatments are provided. Improvement may be expected when the hypertrophic scar was originally produced by excessive endothelial and fibroblastic cell proliferation, as is present in open wounds, burns, and infected wounds. However, little or no improvement can be anticipated if the hypertrophic scar followed uncomplicated healing of a simple surgical incision. Improvement of hypertrophic scars across flexion surfaces such as the antecubital fossa or the fingers requires a procedure such as a Z-plasty to change the direction of the scar.
Pressure may help flatten a potentially hypertrophic scar. It is particularly useful for burn scars. A measured elastic garment or face mask (Jobst) is applied to the scarred area and provides continued pressure that causes realignment and remodeling of the collagen bundles. Pressure should be applied early, continuously, and for 6-12 months. Use of intermittent pressure (eg, only at night) or pressure applied after the hypertrophic scar is established (6-12 months) is of little value.
Additional methods of decreasing the thickness of hypertrophied scars include silicone sheeting applied early and continuously for weeks or months. Laser therapy, such as CO2, pulse dye, and flash lamps, have been examined with no definitive long-term success noted when used alone. Radiotherapy has a place in scar management although it is somewhat controversial due to potential carcinogenesis following procedure and the lack of long-term follow up studies. Cryotherapy has shown some success with small scars only. Interestingly enough, the simple use of paper tape over fresh surgical incisions for several weeks has shown to be a potential preventive treatment.
The first-line treatment for keloids and second-line therapy for intractable hypertrophic scars is still injection of triamcinolone acetonide, 10 mg/mL (Kenalog-10 Injection), directly into the lesion. Corticosteroid injection decreases fibroblast proliferation and collagen synthesis as well as suppressing pro-inflammatory markers. There is some evidence that keloids may respond better to early treatment rather than to late treatment.
Lesions are injected every 3-4 weeks, and treatment should not be carried out longer than 6 months. A general rule of thumb is to inject 10 mg of triamcinolone for each centimeter in length of the scar. The following dosage schedule is used:
For larger lesions, the maximum dose should be 120 mg. The maximum doses for each treatment for children are as follows:
There is a tendency to inject the drug into the scar too often or in too high a dosage—or into the subjacent tissue, which may produce too vigorous a response, resulting in excessive atrophy of the skin and subcutaneous tissues surrounding the lesion and in depigmentation of darker skins. Both of these adverse responses may improve spontaneously in 6-12 months, but not necessarily completely. Cushing symptoms have even been reported with the overuse of corticosteroids for scar management. Topical corticosteroid therapy is of little or no value for substantial scarring but may have some place with more superficial scarring, such as in the case of dermabrasion.
At present, surgical excision is used only in conjunction with intralesional corticosteroid therapy. Excision is usually confined to the larger lesions in which steroid therapy would exceed safe dosages. (The wound is injected at the time of surgery and then postoperatively according to the schedule recommended above.) Care should be taken so that surgical incisions are not extended into the normal skin around the keloid, since the growth of a new keloid may occur in these scars.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree