OUTLINE
Basic Requirements and Considerations for TPN Therapy
Nutrients and Fluid Requirements
I. DEFINITIONS
A. As the name implies, total parenteral nutrition (TPN), or total nutrition admixture (TNA), is intravenous therapy that provides nutrition to patients who cannot take nourishment by mouth.
1. The aim of TPN is to replace and maintain by IV infusion essential nutrients when (and only when) oral or tube feedings are contraindicated or inadequate. It is used only when necessary because of the risks associated with this therapy and the high cost of this treatment.
2. Typical situations when TPN is used include the following:
a. Severely undernourished patients without oral intake for more than 1 week.
b. Severe pancreatitis with intolerance to enteral nutrition.
c. Severe inflammatory bowel disease (Crohn’s disease and ulcerative colitis) exacerbated by nutrients administered via the gastrointestinal (GI) tract.
d. Extensive bowel surgery (i.e., short bowel syndrome).
e. Small- and large-bowel obstruction.
f. Pregnancy (in cases of severe nausea and vomiting).
g. Head-injury patients with no enteral access or with GI dysfunction.
B. “All-in-one,” “3-in-1,” or TNA formulations are TPNs that contain injectable lipid emulsions in the same container with traditional amino acids, dextrose, and electrolytes.
C.Hyperalimentation (HA) is an older term for TPN.
D. Peripheral parenteral nutrition (PPN) is described under Special Topics (section IV) later in this chapter.
II. BASIC REQUIREMENTS AND CONSIDERATIONS FOR TPN THERAPY
A. What to give
1. Basic nutrients and fluid
a. Dextrose—the major source of calories. Each gram of dextrose provides 3.4 kcal.
b. Amino acids—for the protein synthesis required for tissue growth and repair. Each gram of protein provides 4 kcal.
c. Lipid—for required essential fatty acids and as a source of calories. Each gram of lipid emulsion provides 9 kcal, and the glycerol component contributes 1 kcal, so the total caloric contribution is 10 kcal per gram of IV lipid.
d. Basic electrolytes—Na, K, Mg, Ca, phosphate.
e. Vitamins.
f. Trace elements—Cu, Cr, Zn, Mn, Se.
2 Histamine H2-receptor antagonists—to prevent and treat upper-GI, stress-related ulceration.
These medications are often included in TPN formulations.
B. To avoid exceeding normal daily fluid limitations, the nutrients are usually administered as highly concentrated, hypertonic solutions. To give some perspective, approximate osmolarities (in mOsmol/L) of plasma, two large-volume parenteral solutions, and a typical TPN are given here:
Plasma | 300 |
0.9% NaCl | 300 |
5% Dextrose in water (D5W) | 250 |
TPN (central) | 1,800 |
C. Vein damage, which would be caused by giving these highly hypertonic TPN solutions, is minimized by administering the TPN solution through a large-diameter central vein where blood flow is rapid. This enables the TPN solution to be diluted rapidly as it flows into the body.
1. The usual position of the central catheter is the superior vena cava. Because this vessel is near vital organs and blood supplies, catheter placement is verified by radiograph.
2. If a peripheral vein is used for administration of parenteral nutrition, the solution given must be less hypertonic. PPN solutions have osmolarities of approximately 700 to 900 mOsmol/L.
3. Heparin (3,000 units/day) and hydrocortisone (5 mg/L) are often added to PPN formulations to decrease the risk of thrombophlebitis.
III. NUTRIENTS AND FLUID REQUIREMENTS
The values given in this section are the average 24-hour requirements for an adult. See also the TPN example and Table 35.2, TPN Requirement Worksheet, at the end of this chapter. McMahon et al. provide an excellent review of TPN requirements (1). Other good sources of information include the Internet sites of the American Society of Parenteral and Enteral Nutrition (ASPEN) at www.nutritioncare.org and the Baxter Healthcare Corporation at www.nutriforum.com.
A. Body weight
1. Actual body weight (ABW) is most commonly used for calculation of fluid and nutritional requirements. When the actual weight of a patient exceeds ideal body weight (IBW) by 30% (i.e., ABW >130% IBW), an adjusted body weight can be determined for calculation of nutritional requirements. The following equation may be used to calculate adjusted body weight:
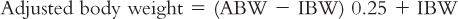
2. For the equations used to calculate IBW, refer to Chapter 9, Evaluating Dosage Regimens.
Note: In the sample calculations for fluid and nutrient requirements given later, a “typical” adult with ABW of 70 kg is used.
B. Fluid requirements
1. General
a. 2,500 to 3,500 mL for an average adult
b. 500 to 2,000 mL for an adult in renal failure (depending on the severity of the disease)
2. Based on body weight (two different methods shown):
a. 30 to 35 mL/kg of ABW
Example: For a 70-kg adult = 30−35 mL/kg × 70 kg = 2,100−2,450 mL
b. 1,500 mL per the first 20 kg, then 20 mL/for each kilogram ABW above the first 20 kg.
Example: For a 70-kg adult = 1,500 mL + (20 mL/kg × 50 kg) = 2,500 mL
C. Protein (amino acid) requirements
1. General: 0.8 to 2.0 g protein (amino acids)/kg ABW
Example: For a 70-kg adult: 0.8−2.0 g/kg × 70 kg = 56−140 g amino acids
2 The amount of amino acids given will depend on the stress level and/or the extent and level of body injury, with 1 g protein/kg given for mild stress and up to 2 g/kg for severe stress.
D. Dextrose
1. Dextrose is the major source of nonprotein kilocalories, but IV lipid is also used.
2. General requirements: 3 to 5 mg/kg/min
Example: 24-hour dextrose requirement for a 70-kg adult:
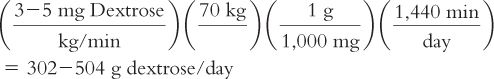
Notice that to furnish this amount of dextrose using a 10% dextrose solution, 3,020 to 5,040 mL per 24 hours would be required, greatly exceeding the normal daily fluid requirements. This is why more concentrated dextrose solutions (20% to 30% dextrose) are required for TPN therapy, which explains why TPN solutions are so highly hypertonic.
E. Lipid injectable emulsions
1. Available as 10%, 20%, or 30% emulsions
a. The 10% product supplies 1.1 kcal/mL, the 20% product 2 kcal/mL, and the 30% product 3 kcal/mL.
b. The osmolarity ranges from 200 to 300 mOsmol/L, with glycerin added to make the lipid emulsions isotonic.
c. The 30% product is indicated only for administration as part of a 3-in-1 or TNA.
2. Uses
a. Originally, lipid injectable emulsions were given in one of two ways:
(1) Administered two to three times per week as a separate IV product, not primarily as a calorie source, but to prevent essential fatty acid depletion (EFAD)
(2) Administered daily
(a) For patients, such as diabetics, who have difficulty tolerating high dextrose loads, IV lipid emulsion was given by constant infusion as a source of calories. It was administered in a separate IV line by using a Y-tubing IV administration setup.
(b) For patients on PPN, IV lipid emulsion was either mixed directly with a concentrated TPN solution or the two IV products were run together using Y-tubing. In this case, the lipid emulsion served as a calorie source while cutting down on the total osmolarity of the solution.
b. Now it is recognized that most patients require daily lipid, so IV lipid emulsion is given on a daily basis to nearly all patients.
(1) IV lipid should comprise 1% to 4% of the total calories delivered to prevent EFAD.
(2) The dextrose, amino acids, electrolytes, and lipid emulsion are often incorporated into one container. These are referred to as all-in-one or 3-in-1 solutions, or TNAs, and these preparations are infused via a central vein. CAUTION: In the spring of 1994, several deaths were reported with patients using these combined solutions. Autopsies showed the presence of precipitated calcium phosphate in the lungs of these patients; apparently the precipitation occurred in the 3-in-1 solutions and its presence was masked by the lipid emulsion. As a result, the U.S. Food and Drug Administration (FDA) has issued guidelines for compounding these solutions (see the FDA Safety Alert in Figure 35.1). An analysis of the factors affecting precipitation of calcium phosphate is given in section IV.C.
(3) When provided on a daily basis, it is recommended to administer ≤ 30% of the total daily calories as IV lipid over a 12- to 24-hour period to avoid immune dysfunction.
3. Dissadvantages, cautions, and precautions with IV lipid emulsions
a. A complication similar to an allergic reaction (chills, chest pain, sensation of warmth) occurs rarely (less than 1%). To check, patients can be started at a slow rate (1 mL/min for 30 minutes) and the product can be discontinued if a reaction occurs. However, this test dose is rarely performed in clinical practice.
b. Lipid emulsion is contraindicated in patients with egg allergies, because the emulsion is stabilized with egg phospholipids.
c. IV preparations containing lipid emulsion cannot be filtered with standard 0.22-μm in-line IV filters, because these would filter out the lipid globules;a 1.2-μm filter is therefore recommended.
d. There is a limit to the amount of calories that can be supplied by lipid; according to the product package insert for IV lipid emulsion, the absolute maximum is 60%. All patients need a certain amount of dextrose per day.
e. Lipid emulsion must be used cautiously in patients who have phosphate restrictions (such as patients in renal failure), because it contains approximately 7.5 mM P per 500 mL.
f. It should be used cautiously in patients with a history of hyperlipidemia.
g. It must be used cautiously in preterm infants, because they have immature hepatic function and therefore have poor clearance of lipid that can accumulate in the lungs. This may be fatal.
h. Questions exist regarding immunosuppressant activity of lipid emulsion when it is given by bolus infusion (i.e., over less than 6 hr). This does not seem to be a problem when IV lipid is given slowly by continuous IV infusion over a 12- to 24-hour period. On the other hand, when IV lipid emulsion is administered as a separate infusion in addition to dextrose and amino acids, it should be infused over no longer than 12 hours; this time limit is recommended to prevent the growth of microorganisms that can be inadvertently introduced into the manufacturer’s original container during IV administration.
4. In 2004, the USP announced the development of Chapter 〈729〉, Globule Size Distribution in Lipid Injectable Emulsions, a proposal to define specific globule size limits in order to promote standardization in the quality of lipid emulsions produced by commercial manufacturers (2).
a. Two methods are identified for analyzing the size and dispersion of lipid globules, a critical factor determining the safety of lipid emulsions.
b. One method uses light-scattering techniques to determine the mean droplet size (MDS); this technique is viewed as a manufacturing parameter (3).
c. The upper limit for MDS is 500 nm or 0.5 μ m.
d. The second method uses the light obscuration or light extinction method to identify the amount of fat globules in the large-diameter tail of globule populations for lipid injectable emulsions; this technique is viewed as a stability parameter (3).
e. The upper limit for this population of fat globules is a volume-weighted percent of fat >5 μm or expressed as PFAT5 < 0.05%.
f. The intent of the PFAT5 limit is to identify coarse dispersions of lipid injectable emulsions that may alter lipid clearance from blood circulation as well as unstable lipid emulsions that may ultimately deposit in the lungs and cause respiratory failure.
g. From a clinical standpoint, studies of compounded TNAs have demonstrated obvious phase separation or “cracking” of formulations with PFAT5 >0.4% (i.e., approximately tenfold higher than the upper limit) (4).
h. In general, final concentrations of amino acids must exceed 4%, dextrose concentrations must be >10%, and lipid emulsion concentrations must be >2% in order to maintain TNA stability (3).
F. Total kilocalories-per-day requirement
1. The general requirement for total kilocalories per day is 25 to 35 kcal/kg ABW.
2. As with the amino acid requirement, the amount of kilocalories per kilogram of ABW will depend on the patient’s stress level, disease state, and level of body injury. This is based on the theory that in certain diseases states, more calories are expended.
3. There is some controversy about increasing the level of kilocalories per kilogram of ABW based on the level of stress—for example, 25 kcal/kg in mild stress, 30 kcal/kg with moderate stress, and 35 kcal/kg in severe stress. As dextrose is the major source of calories, you may ask, “Would there be any problem with giving too much dextrose?” Remember from biochemistry:
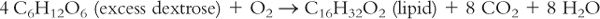
Too much dextrose can result in the following:
a. Lipid deposited in the liver with the result of liver dysfunction.
b. Excess carbon dioxide production with the result of respiratory distress, especially a problem with patients on a ventilator or with chronic obstructive pulmonary disease (COPD). If extra calories are needed for a patient with respiratory problems, IV lipid should be given directly to avoid excess carbon dioxide production.
c. Generally, these problems develop when dextrose administration exceeds 7 mg/kg/min.
G. Electrolytes, vitamins, and trace elements
1. Sodium: The parenteral recommended daily intake (RDI) is determined by clinical need; the approximate usual range is 1 to 2 mEq/kg ABW.
a. Sodium is principally an extracellular cation with no established RDI. Its inclusion in the TPN is based upon clinical need.
b. For example, patients with end-stage liver disease or congestive heart failure or those with iatrogenic fluid overload may require severe sodium restriction.
c. Conversely, patients with large nasogastric fluid losses, high ileostomy or pancreatic fistula outputs, or significant small-bowel losses often require substantial quantities of sodium per day.
2. Potassium:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


