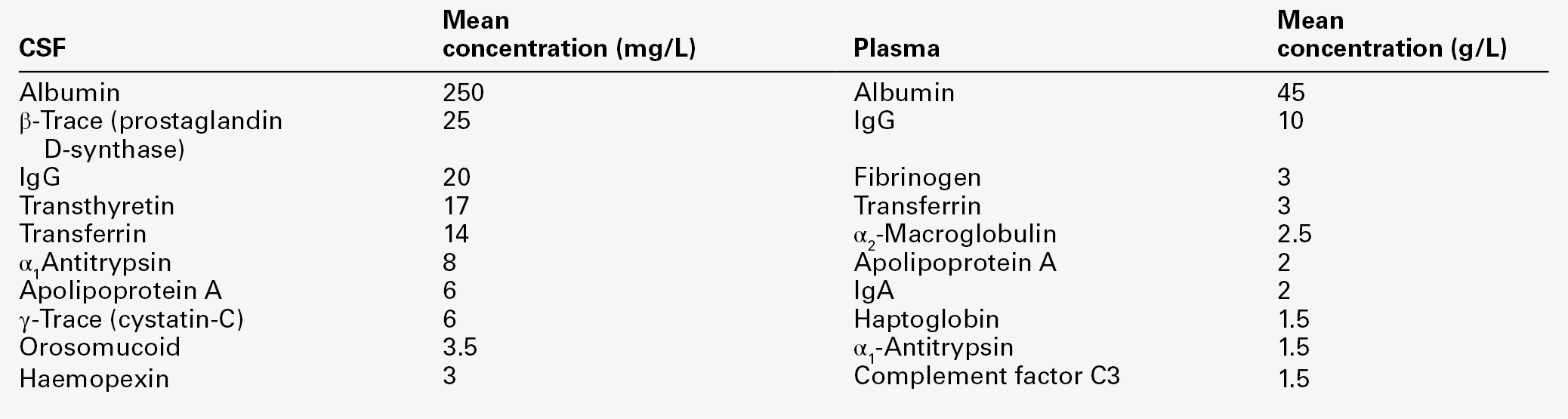
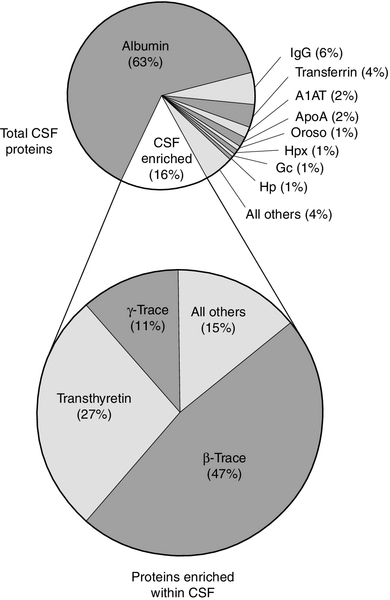
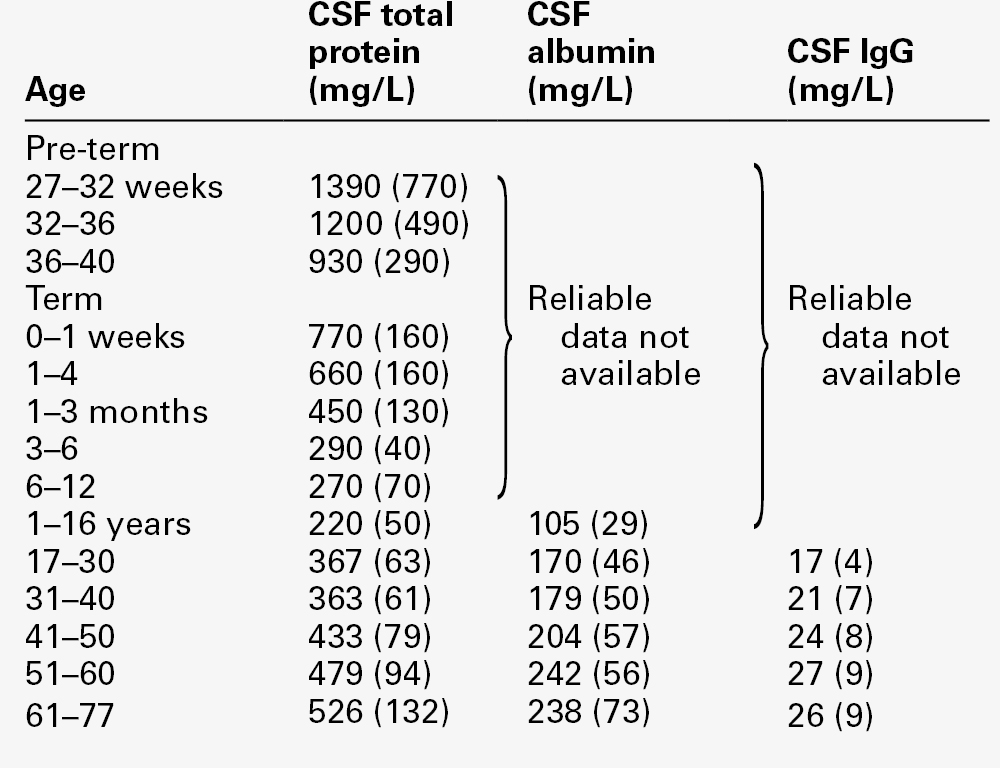
CHAPTER 34 Geoffrey Keir; Carrie Chadwick CHAPTER OUTLINE CEREBROSPINAL FLUID PHYSIOLOGY Analysis of cisternal or ventricular fluid INVESTIGATIONS RELEVANT TO PHYSIOLOGY AND PATHOPHYSIOLOGY Cerebrospinal fluid oto- and rhinorrhoea Non-biochemical investigations BIOCHEMICAL INVESTIGATIONS IN CNS DISORDERS Cerebrospinal fluid analysis in demyelinating diseases Spinal paracentesis (lumbar puncture) was introduced at the end of the 19th century for the therapeutic relief of raised intracranial pressure secondary to tuberculous meningitis. Within a few years, however, it was being used to sample lumbar cerebrospinal fluid (CSF) to aid clinical diagnosis. Analyses that were undertaken in these early days included microscopy for cells and chemical tests to measure reducing substances, protein and chloride. Even today, counting cells and measuring total protein and glucose remain the mainstay analyses routinely carried out on CSF specimens. Yet, modern analytical techniques make possible the quantitative and qualitative detection of a vast range of biochemical substances. It is the purpose of this chapter to assess critically the role of laboratory studies of CSF in the investigation of central nervous system (CNS) disorders. The results of these analyses must be considered both in the clinical context and in conjunction with the findings of other investigations, including neuroimaging and neurophysiological measurements. A few points should be kept in mind when handling CSF. Despite the fact that well-documented procedures exist that will minimize discomfort, including that of post-lumbar puncture headache, the anticipation of a lumbar puncture (LP) is something that causes distress to many patients. This is not helped by the fact that, occasionally, even when carried out by an experienced practitioner, performing a satisfactory LP can prove difficult and uncomfortable and is not without the risk of epidural or subdural bleeding or infection. In many instances, only one spinal tap can be carried out. One reason is that the introduction of a needle into the lumbar sac to sample the CSF can lead to blood contamination of the remaining CSF. This can invalidate the taking of further samples for several days, the exact time being dependent upon the analyte being studied and the degree of blood contamination. There are also ethical reasons against repeating an invasive procedure for trivial reasons. Furthermore, in the very young, the volumes of CSF that can safely be taken are small. Collectively, this places a heavy burden of responsibility on laboratories to make sure that the maximum relevant information is achieved from the available fluid, and that none is wasted or prematurely discarded. Also, the analysis of CSF is multidisciplinary, and it behoves laboratories to ensure that there is the necessary cooperation among pathology specialties. It is strongly recommended that laboratories produce guidelines for doctors indicating sample requirements, and establish procedures to ensure that all CSF samples surplus to immediate analytical requirements are correctly stored. Like all body fluids, CSF is a potentially hazardous material and must be handled appropriately. In the normal adult, the total volume of CSF is about 150 mL. Cerebrospinal fluid is formed at a rate of approximately 500 mL/day, so the fluid is typically exchanged about four times daily (every 6 h). Infants and children have smaller CSF volumes that range from 30–60 mL in a neonate to 100 mL in a pre-teen. While the choroid plexuses that line the ventricles are the major site of CSF production, as much as 30% of CSF is formed by fluid shifts across various vascular beds within the CNS, such as the cerebral capillaries and dorsal root ganglia. Unlike choroidal CSF, interstitial fluid forms throughout the brain and spine, and then diffuses into the CSF. Cerebrospinal fluid formed in the lateral ventricles passes through the third and fourth ventricles into the cisterna magna, from where it circulates out into the cerebral and spinal subarachnoid spaces. The subarachnoid space lies between the two leptomeninges, the outer (arachnoid) mater and inner (pia) mater, which cover the whole brain and spinal canal down to the level of the second sacral vertebra. The flow of CSF from the cisterna magna is mainly upward and outward over the cerebral hemispheres to the main site of reabsorption through the arachnoid villi, which drain into the major dural sinuses. Flow down the cul-de-sac of the spinal cord is relatively sluggish. Thus, while the average turnover time of CSF is ~ 6 h, radiolabelled plasma albumin can continue to equilibrate with lumbar CSF for 1–2 days. Solutes enter the CSF by a variety of processes, including active transport via specific transporter mechanisms present in choroidal epithelial cells, facilitated diffusion and passive diffusion. Cerebrospinal fluid constituents may be derived from plasma, from the metabolic activities of cells normally present in the CNS or from cells and organisms present in the CSF as a result of pathological processes. While the most common fluid obtained for analysis is lumbar CSF, there are occasions when CSF is taken from other regions, for example from one of the lateral ventricles (usually via a ventriculo-peritoneal or ventriculo-extracorporeal shunt), the 4th ventricle (by cisternal puncture) and even from around the cortex. It is extremely important that the anatomical site of the fluid is recorded, as the reference ranges for many constituents vary according to the anatomical source of the CSF. Details of CSF sampling techniques are well described in standard textbooks of practical medical procedures. The initial hydrostatic pressure is between 80 and 180 mmH2O when the patient is in the lateral recumbent position. Ideally, four sequential fractions of CSF should be collected into sterile polypropylene containers. In an adult, a total of 10–12 mL of fluid should be withdrawn, but as little as 3 mL or less in the case of a neonate. The containers must be numbered in the order of collection. Typically, fraction 1, taken into a fluoride tube, is used for glucose (this fraction may also be suitable for protein measurement by some methods), fractions 2 and 3 are used for cell counts and microbiological examinations, i.e. Gram stain and culture, and fraction 4 is used for the assay of specific proteins and oligoclonal bands and, if required, spectrophotometry (in which case the tube must be protected from light from the moment of collection). Blood for glucose and, in the case of spectrophotometry, for total protein and bilirubin should be taken at the same time. Cerebrospinal fluid is normally crystal clear and colourless. The fluid appears turbid when there are more than 200 × 106 white cells/L (200/mm3) or 400 × 106 red cells/L (400/mm3). The presence of bacteria or contamination by epidural fat may also cause turbidity of the specimen. Clot formation may occur when protein concentrations are markedly elevated; where the source of the protein is blood then at least 1 000 000 × 106 red cells/L (106/mm3) are required for clotting to occur. Cerebrospinal fluid may be coloured yellow by bilirubin or, rarely, by carotenoids, red (or more usually pink/orange) by oxyhaemoglobin or brown by methaemoglobin. Yellow discolouration of CSF is called xanthochromia, a term that has often been used to encompass the colours imparted by both bilirubin and oxyhaemoglobin. Because the presence of oxyhaemoglobin and bilirubin is most appropriately detected by spectrophotometry, where this technique is used, the term xanthochromia should be avoided to minimize confusion. The total leukocyte count in normal adult CSF is rarely > 5 × 106/L mononuclear cells (lymphocytes and monocytes). The presence of even one polymorphonuclear leukocyte (neutrophil, polymorph) in the CSF should be a cause for concern in an adult. In neonates, however, the normal cell count is < 30 × 106/L (predominantly polymorphs), with < 10 × 106/L lymphocytes. Changes that occur in the presence of CNS infections form an important part of the initial diagnosis and will be considered later. Erythrocytes are not normally present in CSF. They appear either following trauma to blood vessels during LP or after an intracranial bleed. A traumatic LP occurs when the needle damages a blood vessel while passing through the vascular epidural space, thereby introducing blood directly into the lumbar sac, and is estimated to occur in 15–20% of all LPs. Traumatic contamination complicates interpretation in two ways. • The second is in the interpretation of the CSF white cell count (WCC). Usually an increase in the number of white cells is an indication of infection, but following a traumatic tap, white cells are more likely to be from the contaminating blood. When a CSF leukocytosis is suspected in spite of a traumatic tap, the predicted white cell count can be compared with that observed, as follows: A ratio of observed (O) to predicted (P) WCC > 1 implies CSF leukocytosis. There is a significant overlap of causes for O:P values between 0.75 and 1.0. However, an O:P ratio of < 0.1 is highly predictive in terms of excluding infection. The brain has no significant glycogen store and is therefore wholly dependent upon the blood supply of glucose to satisfy its requirements. Although the brain is only about 5% of the total body mass, it uses 20% of the glucose available from the blood. Cerebrospinal fluid glucose concentrations can only meaningfully be interpreted in relation to the plasma concentration, measured on a sample ideally taken within 15 min of LP. Cerebrospinal fluid glucose is derived solely from plasma glucose and is normally 60–80% of the concentration in the latter, although, for the first six months of life, the CSF glucose concentration may equal that of plasma. The CSF:plasma glucose ratio falls below 0.6 in a number of conditions, and this finding can be used as a diagnostic aid. The most notable reductions are observed in bacterial, tuberculous and some fungal meningitides, and in hypoxia, whereas ratios are usually normal in viral meningitis. The exact cause of the lowered CSF:plasma glucose ratio is still unresolved and will be discussed below. Whatever the cause, a lowered ratio usually indicates a diffuse generalized meningeal disease. A false negative result may be obtained if the patient has been given prior treatment with antibiotics. Glucose is transferred into the CSF by a specific membrane carrier transport system. In the adult, the usual ratio is maintained up to plasma glucose concentrations of 20 mmol/L. For plasma glucose concentrations higher than this, the CSF glucose concentration does not rise further, probably due to saturation of the transport system. In severe hyperglycaemia, therefore, the CSF glucose may seem disproportionately low relative to that in the blood. It is important that this is not misinterpreted as evidence for infection. Furthermore, the CSF typically takes 2–4 h to equilibrate fully with a change in blood glucose. In a diabetic patient who has recently taken either insulin or an oral hypoglycaemic agent, it is therefore possible to have the paradoxical finding of a CSF glucose concentration that is higher than that in a paired blood sample. Cerebrospinal fluid lactate concentration is normally < 2.5 mmol/L; concentrations are controlled independently of those in arterial blood, indicating that it is a product of metabolism within the CNS. An increased CSF lactate concentration is observed in cerebral hypoxia (for example, following a cerebral infarction) and, most notably, in bacterial meningitis, when it is often associated with a decreased CSF glucose concentration. Elevated CSF concentrations of lactate have been demonstrated in patients with inherited disorders of the mitochondrial electron transport chain involving the pyruvate dehydrogenase complex, which give rise to the mitochondrial myopathies. In those with predominantly neurological symptoms, lactate concentrations may be increased only in the CSF. Two-dimensional protein electrophoresis of CSF reveals 200–300 spots, of which about half correspond to 34 unambiguously identified proteins. Nearly all proteins give rise to multiple spots owing to heterogeneity arising from various combinations of glycosylation, phosphorylation and splice variants. Proteins in CSF can be grouped as follows: • those arising from plasma proteins that have crossed the blood–CSF barriers • those synthesized in the brain and secreted into the CSF • those usually present inside CNS cells that have leaked into the CSF following cell damage. Proteins within this group are generally present in only trace amounts. Table 34.1 lists the ten proteins with the highest concentrations in CSF and plasma. Some 80% of the total CSF protein concentration is derived from plasma proteins that have diffused passively across the various blood–CSF barriers. The remainder comprises proteins that are synthesized within the CNS (Fig. 34.1). Some plasma proteins, such as transferrin, undergo receptor-mediated transfer. The cells of the choroid plexus also synthesize transferrin and prealbumin, so the CSF concentration is a function of several factors. In health, two factors influence the CSF concentrations of the individual plasma-derived CSF proteins. One is the degree of permeability of the combined blood–CSF barriers to the protein, which is inversely proportional to the Stokes radius of the protein. The other is the plasma concentration of the protein: in general, the CSF concentration is directly proportional to that in plasma, and inversely proportional to molecular size. FIGURE 34.1 Protein composition of CSF showing those proteins that are plasma derived and those whose synthesis occurs within the CNS. All the frequently measured plasma proteins are present in CSF, demonstrating the lack of an upper limit for molecular size exclusion by the blood–CSF barriers. In health, all CSF albumin and immunoglobulin come from the plasma. Albumin accounts for 50–60% of total lumbar CSF protein and is present at a concentration of about 1/250 of that of the plasma. Permeability of the barrier alters with age; total CSF protein and albumin concentrations reflect this (Table 34.2). The albumin concentration (and thus that of total protein) also increases from ventricle to lumbar regions, reflecting both a change in the permeabilities of the blood–CSF barriers along the CNS axis and the longer time for equilibration of lumbar CSF with plasma. Cerebrospinal fluid total protein concentrations may increase in two circumstances: • when there is reduced flow of spinal CSF, such as occurs with a partial or complete blockage of CSF flow down the spine (e.g. owing to a prolapsed intervertebral disc, an abscess or a spinal tumour). In this circumstance there is increased equilibration of CSF with plasma, often accompanied by an inflammatory increase in permeability. In Froin syndrome, there is complete spinal block of CSF flow, usually due to a spinal tumour; cerebrospinal fluid distal to the block stagnates, and this eventually allows complete equilibration of the CSF with plasma leading to protein concentrations that are similar in both fluids. A comprehensive review of the role of CSF protein analysis in the investigation of CNS disorders is included in the Further reading list. Measurements of total protein, albumin and α2-macroglobulin concentrations, and electrophoresis have all been advocated as investigations appropriate to the assessment of barrier permeability. Total protein has been the most widely used and must be interpreted against age-related reference intervals. Some authors have advocated a correction when blood is present due to a traumatic tap, recommending the subtraction of 10 mg/L for every 1000 × 106 erythrocytes/L. The cell count and protein determination must be performed on the same sample of CSF and there should not be any degree of cell lysis, which would otherwise render the correction void. The correction assumes a normal plasma total protein and haematocrit and, while it should be used with caution, provides an approximate measure of the degree to which the CSF protein concentration may be increased following addition of blood from whatever source. Albumin is a better indicator of barrier dysfunction, for three reasons: • it is a homogeneous protein, unlike total protein, which is a composite of variable proportions of multiple proteins • its concentration has a lesser degree of age dependency than that of total protein. Despite CSF protein concentrations reflecting plasma concentrations, there is generally no discriminatory advantage to be obtained by relating CSF values to those of plasma. The choice between total protein and albumin will depend both on which assay is available at an appropriate analytical standard and cost. Quality assurance programmes indicate similar overall analytical imprecision for total protein and albumin. Experience of using albumin as an alternative to total protein is, as yet, limited. Intrathecal synthesis of immunoglobulins occurs where B lymphocytes are induced to migrate from the blood into the brain. Once these cells are sequestered in the brain, local cytokine production results in clonal expansion and differentiation into plasma cells, which then start secreting immunoglobulins intrathecally. Intrathecal synthesis of immunoglobulins occurs in a wide variety of neurological diseases, but is most commonly associated with multiple sclerosis and other autoimmune conditions, and infections of the CNS. The immunoglobulin most widely studied is IgG, and this is associated mainly with subacute and chronic conditions. The general approach to detecting intrathecal synthesis relies upon the fact that it is possible to calculate the expected CSF concentration for any protein if the plasma concentration of that protein and the integrity of the blood–CSF barriers are known. The integrity of the blood–CSF barrier is given by the CSF/plasma quotient for albumin. If the blood–CSF barriers are intact, the albumin quotient is typically < 7 × 10− 3
Investigation of cerebrospinal fluid
INTRODUCTION
CEREBROSPINAL FLUID PHYSIOLOGY
Formation
Composition
Analysis of cisternal or ventricular fluid
INVESTIGATIONS RELEVANT TO PHYSIOLOGY AND PATHOPHYSIOLOGY
Sampling and pressure
Appearance
Cells
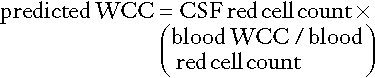
Glucose
Lactate
Proteins
Assessment of blood–brain barrier permeability and reduced fluid flow
Intrathecal immunoglobulin synthesis
Cerebrospinal fluid protein index
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree