OUTLINE
Definitions and Suppository Nomenclature
Selecting the Suppository Base Material
Selecting a Compounding Method
Calculations and Procedures for Compounding Suppositories
Compatibility, Stability, and Beyond-Use Dating
I. DEFINITIONS AND SUPPOSITORY NOMENCLATURE
A. Suppositories: “Suppositories are solid bodies of various weights and shapes, adapted for introduction into the rectal, vaginal, or urethral orifice of the human body. They usually melt, soften, or dissolve at body temperature. A suppository may act as a protectant or palliative to the local tissues at the point of introduction or as a carrier of therapeutic agents for systemic or local action” (1).
B. Other suppository nomenclature
1. As was discussed at the beginning of Chapter 27, in 2002 the USP formed a group to work on simplifying and clarifying dosage form nomenclature. Under the proposed system, the term suppository is used only for rectal administration, and similar dosage forms for administration vaginally or urethrally are termed inserts (2). The definitions in FDA’s 2006 CDER Data Standards Manual have this same distinction and are similar to the proposed USP definitions given here:
a. Suppository:“A solid dosage form in which one or more actives is dispersed in a suitable base and molded or otherwise formed into a suitable shape for insertion into the rectal area to provide local or systemic effect” (2).
b. Insert: “A solid dosage form which is inserted into a body cavity, such as the urethra or vagina. See also SUPPOSITORY, a term used to describe inserts into the rectal region” (2).
2. The proposed nomenclature scheme uses the following format to name preparations: [drug substance] [route of administration] [physical state] [release pattern], so that, for example, the suppositories for Sample Prescription 31.1 would be named: Indomethacin 25 mg Rectal Suppositories. An immediate-release pattern is understood unless otherwise stated.
C. For this chapter, the term suppository will be used in the traditional sense and will include rectal suppositories and vaginal and urethral inserts.
II. USES OF SUPPOSITORIES
A. Suppositories are used when a local effect is needed in the rectum, vagina, or urethra.
B. Rectal and vaginal suppositories may also be used as carriers of drugs for systemic use.
1. Rectal suppositories offer an alternative for the systemic delivery of drugs in patients who cannot take drugs orally. Examples include patients who are unconscious, those who are vomiting or having seizures, and those who have obstructions in the upper gastrointestinal tract.
2. Some drugs that are ineffective orally may be successfully administered rectally or vaginally. Examples include drugs that are extensively metabolized by first-pass effect and drugs that are destroyed in the stomach or intestine. An example of a drug that is administered either rectally or vaginally for systemic effect is progesterone.
C. Compounding suppositories is usually done as a last resort. This is because suppositories are, in general, more difficult to prepare than other dosage forms and because absorption of therapeutic agents from suppositories is relatively unpredictable.
D. As with other dosage forms, suppositories should be compounded extemporaneously only when a manufactured product is not available. This principle is especially true with suppositories containing drugs needed for a systemic effect, because of the unpredictable absorption from suppositories.
III. SELECTING THE SUPPOSITORY BASE MATERIAL
A. To release drug for systemic action or to make drug available for local effect, the suppository base must melt, soften, or dissolve in the body orifice. Of the two most common suppository base materials, polyethylene glycol (PEG) dissolves, while fatty bases, such as cocoa butter and its synthetic substitutes, melt at body temperature. A detailed description of suppository bases and their properties is given in Chapter 24, Suppository Bases. Consult this chapter when selecting a suppository base for a compounded drug preparation.
B. The selection of suppository base material depends both on the intended use (systemic versus local effect) and the route of administration (rectal, vaginal, or urethral). Other factors to consider include patient comfort, and compatibility and stability of components in the base.
1. Patient comfort
a. In general, fatty-type bases are more comfortable for patients than are PEG bases.
b. Fatty bases are bland and nonirritating to the sensitive tissues of the rectum, vagina, and urethra, whereas PEG bases can give a stinging or burning sensation. PEG bases also can cause a defecating reflex when used rectally. These effects can be minimized by adding 10% water to the PEG base and by moistening the suppository with water before insertion (3).
2. Compatibility and stability
a. In most cases, fatty bases are less reactive than PEG bases, so they have fewer compatibility and stability problems with incorporated therapeutic agents.
b. One compatibility problem of fatty bases is the lowering of their melting point with the addition of drugs or other components that form eutectic mixtures with lower melting points. Chloral hydrate in cocoa butter is the classic example of this effect. A small amount of white wax or of cetyl esters wax can be added to overcome this problem; however, adding too much wax may produce a suppository that does not melt at body temperature.
c. Because PEG bases are designed to dissolve rather than melt at the site of action, they can be formulated so that they will not melt on storage, even at fairly warm temperatures. In contrast, fatty bases are formulated to melt at body temperature and are therefore much more sensitive to warm temperatures; they must be stored at controlled room temperature or in the refrigerator.
3. Route of administration
a. Fatty bases are preferred for rectal suppositories because rectal tissues are especially sensitive to the irritating effects of PEG bases. As stated previously, PEG suppositories may also cause a defecating reflex.
b. PEG bases are often preferred over fatty bases for vaginal and urethral suppositories; the vagina and urethra do not have sphincter muscles to prevent leakage from these body orifices, and the oily material of fatty bases is less desirable in this regard.
c. Soft, rubbery suppository bases, such as glycerinated gelatin, are suitable for vaginal administration but are generally not firm enough for rectal or urethral use.
4. Systemic effects
a. In general, systemic absorption of drugs from suppositories is unpredictable. This is due both to the poor environment for absorption in the rectum and vagina (urethral inserts are used solely for local effect) and to the physical-chemical nature of the suppository base material coupled with the properties of the active ingredient(s).
(1) The amount of aqueous fluid in the rectum and vagina is variable but small. This affects the release of drug from the base. Although this influences both local action and systemic absorption, its major impact is on systemic absorption.
(a) Because PEG bases require dissolution for release of the drug from the dosage form, systemic effect depends on the amount of dissolving fluid present in the rectum or vagina. This is part of the rationale for recommending that patients moisten PEG suppositories before insertion; this puts a layer of water in contact with the suppository to help hasten dissolution upon insertion. Even in excess water, PEG bases dissolve slowly, taking up to 1 hour (2).
(b) Although fatty bases melt rather than dissolve, the incorporated drug must partition out of the base into the aqueous medium at the site of action before absorption can occur. This, too, requires a sufficient amount of aqueous media at the administration site.
(2) As was discussed in Chapter 24, fatty bases generally give poor release of hydrophobic drugs. Because many organic drug molecules are water-insoluble and hydrophobic except when present in their ionized salt or water-soluble complex form, this must be considered when choosing both the drug form and the suppository base.
(a) The release of a drug from a fatty, lipophilic suppository base to the aqueous medium in the rectum or vagina depends on the water/base partition coefficient of the drug.
(b) For this reason, from a bioavailability point of view, water-soluble ionized (salt) forms of drugs (which have high water/base partition coefficients) should be used when possible with fatty bases. For example, codeine phosphate is preferred over codeine base for systemic absorption when a fatty suppository base is being used. Some drugs such as acetaminophen and diazepam do not have water-soluble forms and give slow and unpredictable release from most fatty bases.
(c) Some commercially available fatty-acid suppository bases, such as Fattibase, contain surfactants and emulsifying agents that overcome, at least partially, the problem of poor bioavailability of hydrophobic drugs. The free acid indomethacin, which is illustrated in Sample Prescription 31.1, was studied in various suppository bases, and it showed poor release from fatty-acid bases unless surfactants were added to the base material (4).
(3) When formulated with an appropriate blend of polymers, PEG suppositories dissolve in body cavity fluids and release the active ingredient(s), both hydrophilic and hydrophobic drugs. Provided there are sufficient aqueous secretions in the body cavity, PEG suppositories provide more reliable release of hydrophobic drugs from the dosage form than do fatty bases.
b. Suppositories made with bases that contain a dispersing agent, such as silicon dioxide, silica gel, or bentonite, and/or a surfactant or emulsifying agent may break apart more easily and spread more effectively over the target tissue or absorbing surfaces.
c. Because of the unpredictability of release of drug from suppository bases, it is important to monitor the effectiveness of the drug delivery system by frequent monitoring of therapeutic results. Drugs with narrow therapeutic windows should be administered in compounded suppositories only with the greatest of caution.
5. Local action
a. Choice of a base is not as critical when local action is desired, because nearly any base holds the active ingredient in contact with the affected tissue.
b. Suppositories made with bases that contain a dispersing agent and/or a surfactant may break apart more easily, and the active ingredients may be spread more effectively on the target tissue.
c. When an emollient local effect is desired, a fatty base is preferred.
IV. SELECTING A COMPOUNDING METHOD
A. Suppositories are now almost always made by fusion. The method of hand-rolling suppositories, while seldom used, is discussed and illustrated in this chapter because it offers a truly extemporaneous method that can be used in any pharmacy when a customized suppository is needed and the molds and equipment for the fusion method are not immediately available. At one time, suppositories were also made by compression, but this method is no longer used in extemporaneous compounding.
B. Depending on the compounding method selected, special calculations, compounding techniques, and equipment may be required to give accurate doses of suppositories.
C. Hand-rolling suppositories
1. Advantages
a. Hand-rolled suppositories do not require special calculations.
b. Special equipment is not required for this method. A pill roller is useful, but a broad-bladed spatula or any stiff, flat piece of nonreactive material can be used for this purpose.
c. Cocoa butter is the base used for hand-rolled suppositories. It is readily available in bars or push-up tubes from any drug wholesaler. It is also available in grated form from suppliers of compounding ingredients and equipment.
2. Disadvantages
a. Preparing and forming hand-rolled suppositories requires experience and good technique.
b. Even when well made, hand-rolled suppositories do not have the elegant appearance of suppositories made by fusion.
D. Fusion
1. Advantages
a. The fusion method does not require well-developed manual compounding technique.
b. Suppositories made by fusion have an elegant, professional appearance.
2. Disadvantages
a. Special suppository molds are required to make suppositories by fusion.
b. Caution must be used when incorporating drugs sensitive to heat.
c. Because the components are dosed and measured by weight but compounded by volume, density calculations, mold calibrations, or double-casting procedures are required to give accurate doses.
(1) Because suppositories are semisolid at room temperature, most of the base components and active ingredients are solids and are measured by weight.
(2) The components are then combined, melted, and poured into suppository mold cavities. This means that the dosage unit is measured by volume—the volume of the mold cavity. The final amount of drug in a dosage unit therefore depends on two factors: the w/w concentration of active ingredient in the base material and the weight of the mixture contained in each mold cavity. The weight of mixture in the mold cavity depends on the volume of the cavity and the density of the molten mixture.
(3) Consider the following example: The usual volume of a suppository mold cavity is 2 mL. Depending on the density of the melted mixture of ingredients, the weight of a 2-mL suppository can vary considerably. To calculate the weight per suppository, multiply the density (d) or specific gravity (s. g.) of the mixture times the volume of the mold cavity.
For example:

(4) Obviously, the density of the material has a significant effect on the final weight of the dosage unit. To determine the quantity of base and active ingredients(s) to weigh when compounding suppositories by fusion, it is necessary to either calibrate the mold cavities for the desired base or use a double-casting procedure. There are other factors to consider as well. Examples (31.1 through 31.4) are given and discussed in section V. B of this chapter on fusion compounding methods.
V. CALCULATIONS AND PROCEDURES FOR COMPOUNDING SUPPOSITORIES
A. Hand-rolling of suppositories
This method is limited to suppositories made using cocoa butter as the base material, because cocoa butter is the only suppository base that can be molded without the use of heat. The procedure for hand-rolling suppositories is described here and is illustrated with Sample Prescription 31.2.
1. Check the doses of the active ingredients.
2. Check the compatibility and stability of the active ingredients and the formulation.
3. Decide on the desired finished weight per suppository. The usual weight per unit for various suppository types are as follows:
a. Adult rectal: 2 g
b. Vaginal: 2 to 5 g
c. Pediatric rectal: 1 g
d. Male urethral: 4 g
e. Female urethral: 2 g
4. Calculate the quantity of each ingredient needed for compounding the preparation. Make enough material for two extra suppositories to compensate for loss of material during the compounding process.
a. Multiply the dose per unit times the number of units to determine the amount of each active ingredient.
b. Multiply the finished weight per unit times the number of units.
c. Subtract the total weight of active ingredients from the total weight of the suppositories to determine the amount of base (cocoa butter) needed.
5. If the cocoa butter is in a bar or in large pieces, grate or reduce the cocoa butter to very small pieces.
6. Weigh the calculated amount of active ingredient(s) and cocoa butter.
7. Place the active ingredients(s) in a glass mortar and triturate to a fine powder.
8. Add a small amount of cocoa butter and triturate, using pressure to soften and/or liquefy the cocoa butter so that it acts as a levigating agent for the active ingredient(s).
9. Add the rest of the cocoa butter by geometric dilution, triturating and scraping down the sides of the mortar regularly.
10. Using a metal spatula, remove the mass from the mortar and place the material in a clean piece of white filter paper. Put on a pair of clean plastic disposable gloves. Knead the mass in the filter paper until it is pliable but not soft and sticky.
11. While it is still in the filter paper, start to shape the mass into a cylindric pipe by rolling it in the filter paper between your hands (in the same manner as you made “snakes” out of modeling clay when you were in preschool).
12. Put the cylindric mass on an ointment slab and, using a clean pill roller or a broad-bladed spatula, roll the mass into a smooth cylindric pipe. Use the gauge on the ointment slab or a ruler for determining the proper length of the pipe so that equal pieces of appropriate length can be cut. The approximate dimensions per suppository are as follows:
a. 1 to 1½ inches long with a diameter of approximately 3/8 inch for adult rectal and vaginal suppositories
b. Proportionate but smaller for pediatric rectal suppositories
c. 4 inches long by 3/16 inch in diameter for male urethral inserts
d. 2 to 3 inches long by 3/16 inches in diameter for female urethral inserts
13. As you roll the mass, use the pill roller or spatula to keep the ends of the pipe as blunt and square as possible; you may wish to roll the pipe slightly longer than necessary and cut off the irregular ends of the pipe before cutting it into equal pieces. (Remember that you made enough material for two extra suppositories to compensate for loss such as this during compounding.)
14. Using a razor blade, cut the pipe into equal-length pieces. After you cut the first piece, check its weight to be sure that you are in the approximate desired weight range per suppository.
15. Form a point on one end of each suppository. Suppositories should be bullet-shaped for ease of insertion.
16. Weigh each suppository; if necessary, adjust each to the proper weight by slicing thin pieces from the blunt end. The suppositories should each be the target weight (e.g., 2 g ±10%).
17. Wrap each suppository in foil or seal in individual plastic bags.
B. Fusion
1. Suppository molds
a. A wide assortment of suppository molds is available from vendors of compounding supplies. Depending on the company, aluminum molds for rectal and vaginal suppositories are available with either 1- or 2-mL capacity cavities and with 10 to 1,000 cavities per mold. Urethral molds, both male and female, are also available. A wide variety of disposable molds can also be purchased.
b. Although preference for disposable versus aluminum molds depends on the individual using them, the type of suppository base being used, and the intended use of the suppository, in general, aluminum molds give more uniform, accurate dosage units than do plastic disposable molds.
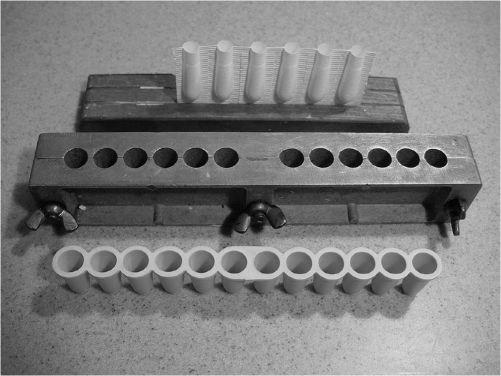
ALUMINUM AND DISPOSABLE SUPPOSITORY MOLDS
c. Mold lubrication
(1) Disposable molds have the advantage of not requiring any prior lubrication because the suppositories are just popped out or the plastic material peeled off at the time of use.
(2) Although aluminum molds usually require lubrication before use, this is relatively easy with the use of spray vegetable oils. Just a light coating is needed; any excess should be wiped off with a tissue.
d. Suppository wrappers
(1) Disposable molds also have the advantage of providing the wrapping material for the suppositories; the suppositories are dispensed in the plastic shell that is used as the mold.
(2) Suppositories that have been made in a nondisposable mold should have a protective covering applied before being placed in the dispensing container. They should either be wrapped individually in aluminum foil or sealed in small polyethylene plastic bags. The foil can be purchased precut in small squares (and it even comes in colors), or the 3” × 3” squares can be cut from a sheet of aluminum foil. Alternatively, small polyethylene bags, zipper-lock or plain, can be purchased for a nominal price.
e. Although aluminum molds have a greater initial investment cost, in the long term they may be less expensive to use than disposable molds.
2. Suppository base materials for the fusion method
a. All four suppository base types that are recognized in the USP (1) may be used with the fusion method. These bases and their properties are discussed in Chapter 24.
b. Melting fatty bases
(1) Microwave ovens are not recommended for melting fatty bases because these devices do not provide the carefully controlled temperature required. Similarly, although these bases could be carefully melted by direct heat on a hot plate, the more controlled temperature provided by a warm-water bath is preferred. This is especially true when melting cocoa butter.
(2) If the base is in a bar or in large solid blocks, grate or shred the base into very small pieces. This increases the efficiency of the melting process. Furthermore, overheating the melt is less likely when there are no large chunks that require melting.
(3) Place the base in a beaker or crucible and carefully heat this on a warm-water bath until the base has just turned to a fluid. If available, use a warm-water bath with a thermostat (such as the one pictured in Chapter 13), because this makes the process of controlled heating much easier.
(4) If the base is cocoa butter, have the water bath temperature at approximately 55°C and melt the base carefully. Melted cocoa butter should maintain an opalescent, creamy appearance with a temperature of approximately 34°C. You can tell visually if the critical temperature has been exceeded for cocoa butter because the fluid changes to a clear, golden color. One recommendation to avoid overheating cocoa butter is to add it in portions to the heating vessel. Then, if the critical temperature is exceeded and the fluid becomes clear, the vessel can be removed from the heat source and extra grated cocoa butter can be added to reduce the temperature of the melt and provide new β-crystals for the desired polymorph.
(5) If a cocoa butter substitute base such as Fattibase is used, follow the manufacturer’s instructions for the heating temperature. For example, the recommended melting temperature for Fattibase is approximately 50°C, and this base should not be heated above 60°C.
c. Preparing and melting PEG and glycerinated gelatin bases
(1) Both PEG and glycerinated gelatin bases can be made using either a microwave oven or a hot plate. In either case, care must be exercised so that the material is not overheated.
(2) Preformulated blends of PEG, such as Polybase, and typical solid PEG base components melt at between 37° and 63°C, but they may be heated up to 100°C without danger of decomposition. The melting points of commonly used PEGs are given in Table 24.1 of Chapter 24.
(3) The formula and method of preparation for glycerinated gelatin suppository base is the same as the pediatric chewable gummy gel base given in Table 26.3 of Chapter 26.
3. Compounding procedures
Four examples are given here to show the necessary calculations and compounding procedures for making suppositories by fusion. These principles are used in Sample Prescriptions 31.1 and 31.3 at the end of this chapter.
Fusion Method When Precalibrating the Suppository Mold:

Procedure:
1. Select the base material for this preparation. In this case, cocoa butter is selected because this is a comfortable base for rectal administration. A synthetic fatty-acid base such as Fattibase would also be acceptable, but a PEG base should not be used because aspirin is reported to be unstable in this type of base (5).
2. Select a suitable suppository mold. If an aluminum mold is used, permanently inscribe an identifying mark on the mold and record this with the calibration information for the base selected. If a disposable mold is used, record the manufacturer and the lot number of the mold with the calibration information.
3. Calibrate the suppository mold for the base being used—cocoa butter, in this example. This is done by carefully melting the grated cocoa butter, pouring it into five suppository mold cavities, trimming and removing each suppository from its mold cavity, and weighing each suppository. Using this weight data, calculate the mean suppository calibration weight for cocoa butter in this mold. Because the density of a base may vary depending on temperature of the melt, note and record the temperature of the melt so that this fusion temperature is used each time this calibration weight is used with this mold and base. In this example, the temperature of the melt is 34°C (the target temperature for melted cocoa butter), and the average weight per suppository is 1.72 g. If another base is used, follow the manufacturer’s instructions for heating temperature.
4. Calculate the amount of active ingredient(s) for the prescription order plus two extras to compensate for loss of material during the compounding process.
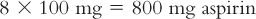
5. Using the suppository calibration weight and the desired number of suppositories, calculate the final weight for all suppositories:
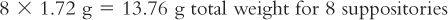
6. Calculate the quantity of cocoa butter needed by subtracting the weight of the active ingredient(s) from the total weight of the eight suppositories.
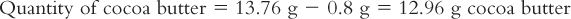
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


