OUTLINE
Definitions and Solution Nomenclature
Advantages and Disadvantages of Solutions
Uses and Desired Properties of Solutions by Route of Administration
Principles of Compounding Solutions
Compatibility, Stability, and Beyond-Use Dating
I. DEFINITIONS AND SOLUTION NOMENCLATURE
A. Solutions: “Solutions are liquid preparations that contain one or more chemical substances dissolved (i.e., molecularly dispersed) in a suitable solvent or mixture of mutually miscible solvents” (1).
B. Traditional pharmaceutical dosage form terms used for solutions are briefly defined and described in the following list. For additional information, see USP Chapter 〈1151〉, Pharmaceutical Dosage Forms (1), and the FDA CDER Data Standards Manual (2).
1. Spirits: These are alcoholic or hydroalcoholic solutions of volatile substances such as camphor and peppermint. The high alcoholic content of a spirit is usually necessary for solubility of the ingredient(s), so addition of water may cause turbidity or precipitation. These solutions should be stored and dispensed in tight, light-resistant containers to retard evaporation of the volatile ingredients and the alcohol and to minimize oxidation of labile active ingredients (1).
2. Tinctures: These solutions contain vegetable materials or chemical substances in alcoholic or hydroalcoholic solvents (1,2). Some tinctures, such as Iodine Tincture USP, are made by direct dissolution (3). Other tinctures, such as those containing vegetable materials, are made by special percolation or maceration processes (1). Compound Benzoin Tincture USP, which is an ingredient in Sample Prescription 27.4, is an example of a tincture made using maceration (3).
3. Aromatic waters: These are clear, saturated aqueous solutions of volatile oils or other aromatic or volatile substances. As with spirits, they should be stored in tight, light-resistant containers (1).
4. Elixirs: This term is commonly used for oral solutions that use a sweetened hydroalcoholic vehicle (1). The CDER Data Standards Manual defines an elixir as, “a clear, pleasantly flavored, sweetened hydroalcoholic liquid containing dissolved medicinal agents” (2).
5. Syrups: Oral solutions that contain a high concentration of sucrose or other sugars are often called syrups, but this term is also used in a more general sense to describe sweet, viscous, oral liquid preparations, including suspensions (1,2).
6. Lotion: Although this term has been used for a variety of topical dosage forms including suspensions, emulsions, and solutions (1,2), the 2006 CDER Data Standards Manual states that the current definition is now limited to liquid emulsions for external application to the skin (2).
7. Otic (ear), ophthalmic (eye), nasal (nose): Words designating the intended route of administration have also been part of the traditional nomenclature for solutions (1).
C. Understanding solution nomenclature
1. As can be seen from the preceding examples, pharmaceutical dosage form terminology is a complex conglomerate; it is the result of historical tradition plus the need to specify in the name of a preparation the physical system (e.g., solution, suspension), the intended route of administration, and, when applicable, the solvent system.
a. Tradition: This has given us such names as spirit, tincture, elixir, lotion, and syrup. Unfortunately, because they just evolved through usage, these terms are not systematic, and most categories are neither all-inclusive nor mutually exclusive. For example, both peppermint spirit and lemon tincture (i) are solutions, (ii) have similar uses, (iii) are made from plant materials, (iv) have volatile components, and (v) have hydroalcoholic solvent systems; however, one is called a spirit and the other a tincture. On the other hand, the term syrup has been used for a wide array of oral liquids, including two different physical systems—solutions and suspensions.
b. Physical system: Identification of the physical system seems simple but terms such as solid, solution, and semisolid are often in sufficient to adequately describe the dosage form. Terms such as tablet, tincture, and cream are needed, and these terms must be specific and well-defined.
c. Route of administration: Solutions are the most versatile of all dosage forms and may be used for nearly any route of administration. The intended route of administration does impose on the solution certain necessary properties, such as sterility for injections, isotonicity for ophthalmic solutions, and palatability for oral solutions. For example, potassium chloride oral solution and potassium chloride injection both contain the same active ingredient, both are made by simple dissolution, and both are aqueous solutions, but their different intended routes of administration require distinctive components and processing controls that result in very different preparations. Therefore, route of administration is an important part of each preparation’s name.
d. Solvent system: The importance of identifying the solvent system can be seen with two official topical solutions: Iodine Topical Solution and Iodine Tincture (3). Pharmaceutically, both are solutions, both are used topically, and both contain 2% iodine, but the fact that one is an aqueous solution and the other has a solvent system containing nearly 50% alcohol makes them very different, and the name of each preparation must reflect this.
2. The numerous dosage form terms and their ambiguity have been sources of confusion among prescribers, pharmacists, and pharmacy technicians, but tradition is a powerful force that often works against needed change. Fortunately, in the relatively recent past, two trends—use of computer technology and globalization—have provided some impetus for the development of a more systematic approach to pharmaceutical dosage form nomenclature.
a. Simple and standard drug dosage form terminology is essential for the efficient (and cost-effective) use of computer technology by government agencies in the drug product approval and monitoring process and by users of patient electronic health information for delivery and monitoring of, and payment for, drug therapy. Impacted organizations and agencies such as USP, the FDA, Health Level Seven (HL7), and the National Committee on Vital and Health Statistics (NCVHS) have been working toward the goal of standardized nomenclature for dosage forms (4).
b. Standardization of terminology is also important for global harmonization of standards. USP and HL7 have been working cooperatively with the International Conference on Harmonization (ICH) on international standards for dosage form nomenclature (4).
c. In 2002, USP formed a group to create a taxonomic scheme that would logically categorize dosage forms and simplify and clarify dosage form nomenclature. Under a system proposed by this group, a dosage form for a drug substance would be identified by the name of the drug, the route of administration (e.g., oral, topical), and the physical system (e.g., tablet, solution, suspension). When appropriate, the release pattern (immediate, extended, etc.) would also be included (4). For example, as of July 2007, Calamine Lotion USP became Calamine Topical Suspension USP (3). The proposed system would eliminate some traditional names such as elixir and spirit. For more information on this project, see USP’s Pharmacopeial Forum 29, Sept–Oct 2003 (4).
d. Development and acceptance of and transition to any new nomenclature system will undoubtedly take years, and it will require the understanding and cooperation of all the affected constituencies. Therefore, it is important for health care workers, and particularly pharmacists and pharmacy technicians, to understand proposed dosage form taxonomy and nomenclature but also to recognize the various traditional terms used to describe dosage forms. The definitions and descriptions in this section should help in this regard.
II. ADVANTAGES AND DISADVANTAGES OF SOLUTIONS
A. Advantages
1. Because solutions are molecularly dispersed systems, they offer these advantages:
a. Completely homogenous doses
b. Immediate availability for absorption and distribution
2. Solutions also provide a flexible dosage form.
a. They may be used by any route of administration.
b. They can be taken by or administered to patients who cannot swallow tablets or capsules.
c. Doses are easily adjusted.
B. Disadvantages
1. Drugs and chemicals are less stable when in solution than when in dry, solid form.
2. Some drugs are not soluble in solvents that are acceptable for pharmaceutical use.
3. Drugs with objectionable taste require special additives or techniques to mask the taste when in solution.
4. Because solutions are bulkier and heavier than dry, solid dosage forms, they are more difficult to handle, package, transport, and store.
5. Oral solutions in bulk containers require measurement by the patient or caregiver. This is often less accurate than individual solid dosage forms, such as tablets and capsules.
III. USES AND DESIRED PROPERTIES OF SOLUTIONS BY ROUTE OF ADMINISTRATION
A. Oral solutions (1)
1. Oral solutions are liquid preparations intended for oral administration.
2. They contain one or more therapeutically active ingredients dissolved in water or a water-cosolvent system. Solutions are most often made by direct dissolution. Factors that affect this process are described later in this chapter. Sample Prescriptions 27.2 and 27.8 illustrate principles and techniques used in making oral solutions.
3. Oral solutions may contain inactive ingredients to improve their palatability, stability, and/or aesthetic appeal. Examples of such ingredients include flavors, sweetening or coloring agents, viscosity-inducing agents, buffers, antioxidants, and preservatives.
4. As described earlier, syrups are oral solutions containing high concentrations of sugars. Oral solutions may contain other polyols, such as glycerin or sorbitol, which prevent “cap-lock” by inhibiting crystallization of the sugars in the cap and adjacent areas of the container. Depending on the polyol, these additives may also serve as sweetening agents, preservatives, cosolvents, and viscosity-inducing agents to improve “mouth feel.”
1. Topical solutions are intended for topical application to the skin.
2. They are usually aqueous but may also contain other solvents, such as alcohols and/or polyols or other solvents approved for topical use.
3. They may also contain such additives as preservatives, antioxidants, buffers, humectants, viscosity-inducing agents, colors, or scents. Preparations in Sample Prescriptions 27.1 and 27.4 through 27.6 are examples of topical solutions.

APPLICATOR BOTTLE
4. Specialized containers for topical preparations are available from vendors of compounding supplies. Bottles with glass applicators, with dauber or roller tops, with sprayer assemblies, and with specialized spout or disc caps are convenient administration aids for topical solutions.
C. Otic solutions (1)
1. Otic solutions are intended for instillation in the outer ear.
2. The vehicle may be water or glycerin or a cosolvent system containing water, alcohol, and/or polyols.
3. Otic solutions may also contain such additives as preservatives, antioxidants, buffers, viscosity-inducing agents, or surfactants. Sample Prescription 27.7 illustrates the use of several additive ingredients in an otic preparation.
4. Bottles with dropper closures are available to facilitate administration of otic solutions.

DROPPER BOTTLE
D. The solutions in the following list are sterile preparations and therefore are discussed in the chapters in Part 6, Sterile Dosage Forms and Their Preparation.
1. Ophthalmic solutions are sterile, particle-free solutions formulated for instillation in the eye (1).
2. Nasal solutions are sprayed or instilled into the nasal cavity. Although they are not specifically mentioned as a class in USP Chapter 〈1151〉 (1), there are official USP nasal solutions such as Naphazoline Hydrochloride Nasal Solution (3). Nasal solutions are most often used for local action, but they may also be used for systemic effect (2).
3. Inhalations are drugs, solutions, or suspensions administered either by the nasal or oral route with the respiratory tract as the intended site for local effect or for systemic absorption of an active ingredient (1,2).
4. Irrigations are solutions used to soak, flush, or irrigate open wounds or body cavities, such as the bladder (1,2).
5. Injections are parenteral preparations injected through the skin or a boundary membrane or directly into a blood vessel, muscle, organ, body cavity, or other tissue (1,2).
IV. PRINCIPLES OF COMPOUNDING SOLUTIONS
A. When making a solution of a drug or chemical, consider the following:
1. Will the drug or chemical dissolve in the desired solvent?
2. How long will it take to dissolve the drug or chemical?
3. Will the drug or chemical stay in solution?
4. Will the drug or chemical be stable in solution? For how long?
5. Is a preservative needed to prevent the growth of microorganisms inadvertently introduced at the time of preparation or during use by the patient?
B. Questions 1 and 2 concern the making of solutions (that is, dissolving drug or chemical in a solvent). Methods and factors affecting dissolution are discussed here. Questions 3 through 5 are considerations of stability and compatibility of drug preparations once they are made. These topics are the focus of Chapter 37, Compatibility and Stability of Drug Products and Preparations, and Chapter 16, Antimicrobial Preservatives, but appropriate consideration of these issues is illustrated in each of the sample prescriptions in this chapter.
C. Will the drug or chemical dissolve in the desired solvent?
1. Dissolution of solids
a. To make a solution of a solid in a solvent, the solid must be sufficiently soluble in that solvent. Obviously, a solid will not dissolve above its solubility.
b. Useful pharmaceutical solvents
(1) Water is the most commonly used and most desirable solvent.
(2) Others common solvents include alcohol (i.e., ethanol), isopropyl alcohol, glycerin, propylene glycol, polyethylene glycol 400, and various oils. These and other USP solvents are described in Chapter 15, Pharmaceutical Solvents and Solubilizing Agents.
(3) Some solvents, such as isopropyl alcohol, are approved for topical solutions but may not be used internally because of their systemic toxicity.
c. When predicting the solubility of a solid in a given solvent, very generally the old saying “Like dissolves like” is a useful guide, where like refers to similarity of molecular structure.
d. Usually more precise information on solubility is required, so the first step in making a drug solution is to check the solubility of the drug. Useful references for this purpose include The Merck Index and Remington:The Science and Practice of Pharmacy.
Boric acid 10%
Purified water qs ad 60 mL On checking the solubility of boric acid, the compounder finds it to be 1 g/18 mL water, or approximately 5%. This preparation cannot be made because the prescribed concentration, 10%, is above the solubility of boric acid in water.
(1) Remember that solubility is given in grams of solute per milliliter of solvent, not per milliliter of solution, so unless you know the density of the saturated solution, you cannot know the precise amount of solution that will result. Therefore, the 5% noted in the preceding example is a rough estimate.
(2) Useful quantity information on saturated solutions of many chemicals and drugs can be found in the Saturated Solutions table in the Miscellaneous Tables section of older editions (13th and prior) of The Merck Index.
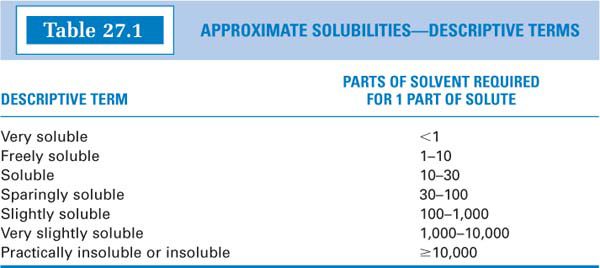
(3) Many times, solubility is given in descriptive terms, such as soluble, slightly soluble, and sparingly soluble. The numerical equivalents of these terms can be found in the USP (5) and other references and are shown in Table 27.1.
e. If possible, always dissolve the drug in pure solvent. For example, although Syrup NF contains a lot of water, these solvent molecules are tied up through hydrogen bonding with the sucrose and are unavailable for the purposes of interacting with and dissolving additional solute. This principle is illustrated with Sample Prescription 27.2.
f. Be careful when using hot water to dissolve drugs or chemicals (a useful technique to speed up dissolution), because the drug may precipitate when the preparation cools to room temperature if its concentration is above its solubility at room temperature. In the example with boric acid, the solubility of boric acid is 1 g/4 mL of boiling water, or approximately 25%. The 10% solution could easily be made using hot or boiling water, but some of the boric acid will precipitate out on cooling to room temperature.
g. If the solution is to be stored or used at a temperature other than room temperature (e.g., in the refrigerator), the solubility of the drug at that temperature must be considered.
h. Following are several useful compounding strategies when a drug solution is prescribed but the desired concentration is above the drug’s solubility:
(1) Make as a suspension. You may need to add a suspending agent, as described in Chapter 28, Suspensions. Remember to use a “Shake Well” label.
(2) Use a different solvent or a cosolvent system. To calculate an estimate of the volume fraction of each solvent needed in a cosolvent system for the drug, use the equation:
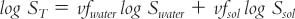
where:

Use of this equation is illustrated with Sample Prescription 27.5 and is discussed in the section on cosolvents in Chapter 37.
(3) As described in Chapter 15, Pharmaceutical Solvents and Solubilizing Agents, cyclodextrins are sometimes used to enhance the solubility of poorly water-soluble drugs. Because the “guest” drug molecule is encapsulated in the cavity of one or more cyclodextrin molecules, cyclodextrins are used in molar ratios of 1:1, 2:1, 3:1, etc., with the target drug. Therefore, when using cyclodextrin to solubilize a drug for a compounded preparation, start with a molar ratio of 1:1 cyclodextrin to drug. If the drug is not completely dissolved, additional cyclodextrin may be required to satisfy a higher molar ratio.
A compounding student attempted to make a published formula that contained progesterone (MW = 314) 20 mg, β-cyclodextrin (MW = 1,311) 62 mg, Purified Water qs ad 1 mL. At this concentration of β-cyclodextrin, the progesterone failed to dissolve completely, but complete dissolution was achieved with 84 mg of β-cyclodextrin, consistent with a 1:1 molar ratio as calculated here:
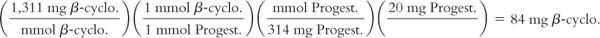
(4) Decrease the concentration of the prescribed drug or chemical. Two different examples of this are illustrated in Sample Prescriptions 27.2 and 27.4. For systemic medications, the volume of the dose to be administered must be adjusted to give the prescribed amount of drug per dose.
(5) In all cases where changes are required, consult with the prescriber.
2. Miscibility of liquids
a. Solubility of solids in liquids has a counterpart with the miscibility of liquids with other liquids. When two liquids are completely soluble (i.e., molecularly dispersed) in each other in all proportions, they are said to be miscible. Some liquid pairs are partially miscible, which means that they are soluble in each other in definite proportions. Immiscible liquid pairs are imperceptibly soluble in each other in any proportion. Whereas you know that oil and water don’t mix (i.e., they are immiscible), consider the miscibility of the following liquid pairs:
b. Obviously miscibility is not always easily predicted. Therefore, if you do not know the miscibility of two liquids, consult a suitable reference, such as The Merck Index or Remington:The Science and Practice of Pharmacy.
c. Following are several useful compounding strategies when it is necessary to combine immiscible liquids.
(1) Make an emulsion by adding an emulsifying agent. Be sure to use a “Shake Well” label. This is described in Chapter 29, Liquid Emulsions.
(2) Use a different solvent or an appropriate cosolvent system. For example, if you need to make a solution that contains alcohol and an oil, use castor oil rather than cottonseed or another vegetable oil.
(3) In all cases in which changes are required, consult with the prescriber.
D. How long will it take to dissolve the drug
In other words, what is the rate of dissolution? In practical terms, what we often want to know is, how can we speed up the rate of dissolution? This can be analyzed in terms of the Noyes-Whitney equation, which was given in Chapter 25, Powders, and which is repeated here.
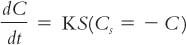
1. From Fick’s First Law of Diffusion, it can be shown that the dissolution rate constant, K, is equal to D/hV, where D is the diffusion coefficient, h is the thickness of the unstirred layer around the particle, and V is the volume of the solvent into which the drug is dissolved.
2. The diffusion coefficient D is actually composed of several factors expressed in the Stokes-Einstein equation given here. Knowledge of these factors will help the compounder to understand conditions that can be changed or controlled to increase rate of dissolution.
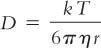
where:

From the Noyes-Whitney and Stokes-Einstein equations and Fick’s First Law of Diffusion, it can be seen that some factors can be controlled or modified to increase the rate of dissolution and some cannot.
dC/dt, the rate of dissolution, is dependent on:
a. T: Temperature is an important factor that can be altered by the compounder. As temperature increases, D increases, so diffusion and the rate of dissolution increase. In practical terms, we can use warm solvents or can heat solutions to increase the rate of dissolution. This is illustrated with Sample Prescription 27.7. Care must be exercised when using heat, because increasing the temperature also increases the rate of degradation of drug molecules.
b. η: Increasing the viscosity of the medium has the opposite effect on diffusion and dissolution rate; increasing viscosity decreases the diffusion coefficient and the rate of dissolution. As a result, drugs dissolve more slowly in viscous vehicles. For this reason, when possible, first dissolve drugs in pure, low-viscosity solvents such as water or alcohol; then add the more viscous necessary liquids, such as glycerin, syrups, or gels. This is illustrated in Sample Prescription 27.2.
c. r: Even though we cannot control the radius of the drug molecule, it is important and helpful to understand how it affects diffusion and the rate of dissolution. The larger the radius (r), the smaller D becomes and the slower the rate of dissolution. This means that, all other things being equal, large drug molecules dissolve more slowly than do smaller molecules. This is especially important when working with macromolecules, such as erythromycin lactobionate and amphotericin B. When making solutions of these drugs, it is necessary to give them sufficient time to dissolve. This is illustrated with Sample Prescription 27.6, a clindamycin topical solution. This factor will have increasing importance as pharmacists and pharmacy technicians handle more peptide and protein drugs, because these are very large molecules.
d. The factor h, which is the thickness of the unstirred layer around the particle, can be affected by stirring. The dissolution rate is faster if the drug-solvent-solution system is agitated or stirred. By stirring, the dissolved drug molecules are moved away from the surface of the solid to the bulk of the solution. This has the effect of decreasing h, which increases K and therefore increases the rate of dissolution.
e. The surface area of the solid, S: As was discussed in Chapter 25, Powders, for a given weight of solid, the surface area of a solid increases as the particle size decreases; therefore, the smaller the particle size, the larger the surface area and the faster the rate of dissolution.
(1) Although important, this principle has limited practical application in modern compounding situations. Most drugs and chemicals are now purchased in a fine state of subdivision. Unless a solid ingredient is in large pieces, any mechanical manipulation, such as trituration, by the pharmacist has only a minor effect on the rate of dissolution. The amount of time saved in speeding the rate of dissolution by decreasing the particle size is usually more than offset by the time lost in the extra steps of weighing, triturating, transferring, and reweighing before dissolving the drug or chemical.
(2) For a few drugs, such as sulfurated potash (used to make White Lotion, as shown in Sample Prescription 28.3), that are available in large chunks or “rocks,” particle size reduction is useful for increasing the rate of dissolution.
f. The solubility of the solid, Cs: Although the solubility of the solid is a given property of the drug, it is important to know that poorly soluble drugs may dissolve slowly.
g. C, the concentration of the drug or chemical in solution at time = t. As the solution approaches saturation, the quantity (Cs − C) gets smaller and smaller until Cs = C. At this point, (Cs − C) = 0, saturation is reached and dissolution stops. As saturation is approached, the rate of dissolution may become very slow. This is one reason for making saturated solutions ahead of time, because getting that last little amount to dissolve may take a long time. Some pharmacies maintain stock bottles of saturated solutions with excess drug or chemical on the bottom of the vessel and then decant the saturated solution when it is needed.
V. COMPATIBILITY, STABILITY, AND BEYOND-USE DATING
A. Physical stability of the system: The major concern with regard to physical stability of a solution is precipitation of a soluble component. Change in temperature, evaporation of solvent, or addition of another drug or chemical to a solution may result in precipitation of a drug or chemical from solution. This subject is discussed in section III. D. of Chapter 37, Compatibility and Stability of Drug Products and Preparations.
B. Chemical stability of the ingredients
1. As stated in section II of this chapter, drugs and chemicals are less stable when in solution then when in drug, solid form. For ingredient-specific information, check references such as those listed in Chapter 37. Examples are illustrated with the prescription ingredients in each of the sample prescriptions at the end of this chapter.
2. USP Chapter 〈795〉 recommends a maximum 14-day beyond-use date for water-containing liquid preparations made with ingredients in solid form when the stability of the ingredients in that specific formulation is not known. This assumes that the preparation will be stored at controlled cold temperature (e.g., under refrigeration) (6). While storage in the refrigerator works well for oral liquids, topical preparations are usually stored at room temperature, and the beyond-use date may need adjustment to compensate for this higher storage temperature. Concerns for chemical stability of labile drugs may necessitate greater limits on beyond-use dates; Sample Prescription 27.1 illustrates this consideration.
3. While technically it is very easy to make solutions, care should always be used in checking and verifying the stability and the beyond-use date of the compounded preparation.
C. Microbiological stability: The USP states that for oral solutions, antimicrobial agents are generally added to protect against bacteria, yeasts, and molds (1). Preservatives should also be considered for topical solutions. If antimicrobial ingredients are prescribed as part of the formulation, extra preservatives are not needed. Antimicrobial agents and their proper use are discussed in Chapter 16, and consideration of preservatives is presented in each of the following sample prescription formulations.
CASE: Ashley Fingerhut is a 110-lb, 5′1″-tall 20-year old female patient who has a painless ulcer on her right hand. She noticed a red papule about 2 months ago and initially self-treated it with various OTC topical creams. When it did not improve and, in fact, ulcerated, she consulted with her pharmacist Jack Jones, who suggested she see her physician. Dr. Attles did a culture of the lesion and has now diagnosed Sporohiza schenckii, which Ashley probably got while working with rose bushes at her summer job at a local greenhouse. Dr. Attles has prescribed a potassium permanganate wet dressing and oral itraconazole 100 mg bid for 3 months.

MASTER COMPOUNDING FORMULATION RECORD
NAME, STRENGTH, AND DOSAGE FORM OF PREPARATION: Potassium Permanganate Topical Solution 0.017%
QUANTITY: 180 mL | FORMULATION RECORD ID: SN001 |
THERAPEUTIC USE/CATEGORY: Germicide/antifungal | ROUTE OF ADMINISTRATION: Topical |
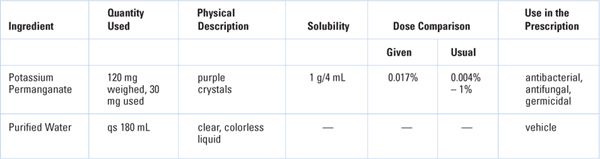
COMPATIBILITY–STABILITY: With regard to physical stability, this solution should be very stable because Potassium permanganate is very water soluble, and this is a very dilute solution. Chemical stability is a very different matter. Potassium permanganate is a very strong oxidizing agent and is not very stable chemically. It can be explosive and flammable if mixed with organic substances such as lactose and glycerin. While it is more stable in aqueous solution, even in this form it has limited stability. This is apparent as solutions turn from purple to brown with the formation of manganese dioxide. With respect to microbiological stability, no added preservative is needed for this solution, because potassium permanganate is an antimicrobial agent.
PACKAGING AND STORAGE: This solution should be dispensed in a tight, light-resistant container. Because this is a topical preparation, controlled room temperature will be the recommended storage condition.
BEYOND-USE DATE: Although it would be possible to use the USP Chapter 〈795〉 recommended maximum 14-day beyond-use date for compounded water-containing liquid formulations prepared from ingredients in solid form (6), use a more conservative 7-day dating because of the labile nature of potassium permanganate.
CALCULATIONS
Dose/Concentration: Concentration okay for intended use
The 1:6,000 ratio strength may be expressed on the label as a w/v%:
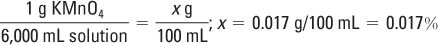
Ingredient Amounts
Potassium permanganate (KMnO4):
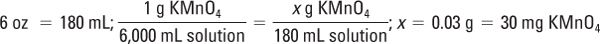
This amount is below the MWQ for the class III torsion balance used for compounding this preparation; a solid–water aliquot is needed. Remember: You may not use any organic diluent (lactose, alcohol, etc.) for potassium permanganate.
Weigh 120 mg KMnO4 and qs ad 12 mL with Purified Water:

Because 30 mg is needed, measure 3 mL of this solution: 10 mg/mL × 3 mL = 30 mg
MSDS AND SAFETY AND PERSONAL PROTECTIVE EQUIPMENT: Review MSDS for potassium permanganate. Don a clean lab coat, disposable gloves, and safety glasses. Use extreme caution in working with the potassium permanganate; it is a strong oxidizing agent and can be explosive and flammable when mixed with organic materials. It is corrosive and causes burns to any area it contacts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Alcohol and water?
Alcohol and water? Glycerin and water?
Glycerin and water? Glycerin and alcohol?
Glycerin and alcohol? Glycerin and mineral oil?
Glycerin and mineral oil? Alcohol and mineral oil?
Alcohol and mineral oil? Alcohol and cottonseed oil?
Alcohol and cottonseed oil? Alcohol and castor oil?
Alcohol and castor oil? Cottonseed oil and mineral oil?
Cottonseed oil and mineral oil? Castor oil and mineral oil?
Castor oil and mineral oil?