OUTLINE
Compatibility, Stability, and Beyond-Use Dating
I. DEFINITIONS
A. Capsules: “Capsules are solid dosage forms in which the drug is enclosed within either a hard or soft soluble container or ‘shell.’ The shells are usually formed from gelatin; however, they also may be made from starch or other suitable substances” (1).
B. Lozenges:“Lozenges are solid preparations, which are intended to dissolve or disintegrate slowly in the mouth. They contain one or more medicaments, usually in a flavored, sweetened base. They can be prepared by molding (gelatin and/or fused sucrose or sorbitol base) or by compression of sugar based tablets…. They are usually intended for treatment of local irritation or infections of the mouth or throat but may contain active ingredients intended for systemic absorption after swallowing” (1).
The definition of lozenge in the FDA CDER Data Standards Manual is similar, but it adds, “a lollipop is a lozenge on a stick” (2).
C. Pastilles:USP 31 states that the term pastille is often used for a subclass of lozenges—that is, molded lozenges (1). Although some references make no distinction among lozenges, pastilles, and troches, traditionally pastilles were soft lozenges containing medicament in a transparent glycerinated gelatin base or a base of acacia, sucrose, and water; they usually were flavored and colored to match the flavor (3). Although the proposed new USP nomenclature does not include the traditional term pastille (4), the 2006 CDER Data Standards Manual does list pastille as a dosage form and defines it as “an aromatic preparation, often with a pleasing flavor, usually intended to dissolve in the mouth” (2). (See the introduction to Chapter 27 for a discussion of proposed revised USP nomenclature.)
D. Troches: Although some references do not distinguish between lozenges and troches (2,5), USP 31 states that the term troches is often used for the subcategory compressed lozenges (1,4), and the CDER Data Standards Manual defines a troche as “a discoid-shaped solid containing the medicinal agent in a suitably flavored base” (2).
E. Molded tablets:“Molded tablets are prepared from mixtures of medicinal substances and a diluent usually consisting of lactose and powdered sucrose in varying proportions. The powders are dampened with solutions containing high percentages of alcohol…. The dampened powders are pressed into molds, removed, and allowed to dry” (1).
II. CAPSULES
A. Uses: As stated at the beginning of Chapter 25, powders for internal use are often formulated into capsules. Hard-shell capsules offer a customized dosage form that can be made easily and conveniently in the pharmacy. Because the quantity of drug formulated into capsules can be measured accurately, this system is especially ideal for potent drugs and chemicals.
B. Hard-shell capsules
1. Empty hard-shell capsules are available in sizes that range from the largest size, 000, to the smallest size, 5. Larger bolus capsule shells are also available for veterinary use in large animals.

RANGE OF CAPSULE SIZES
a. Size 00 (double zero) is usually the largest capsule size used orally for humans. For some patients, even size 00 capsules are too large to swallow.
b. Size 000 (triple zero) capsules are sometimes used to encapsulate medication for rectal or vaginal use. The capsule is then used like a suppository. The capsule should be moistened with lubricating jelly or water before insertion.
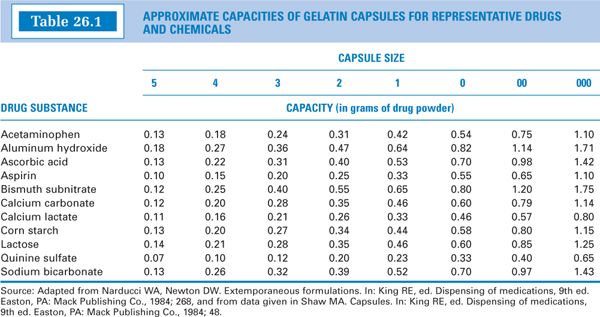
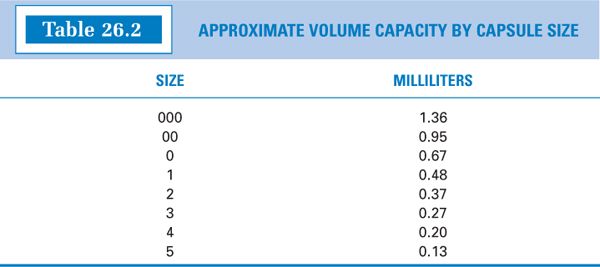
2. The approximate capacities, by capsule size, for representative drugs and chemicals are given in Table 26.1. Notice that the weight capacity for a given capsule size is highly dependent on the density of the powder. The approximate volume capacities in milliliters are given in Table 26.2.
3. Empty hard-shell capsules may be purchased from vendors of compounding supplies. They are available as clear, colorless gelatin capsules and in a variety of colors. Some capsule shells are made opaque by the addition of titanium oxide. These are especially useful when it is necessary or desirable to conceal the powder contents, such as when dispensing powders with an unappealing appearance or when making capsules for blind studies.
4. In addition to gelatin, capsule shells may also contain dispersing agents, hardening agents such as sucrose, or preservatives. A certain percentage of water, usually 10% to 15%, must be present in hard-shell capsules (1). Lack of adequate moisture in these shells causes them to be hard and brittle, which makes them difficult to work with and which may affect their dissolution and bioavailability. For this reason, it is advisable to store capsules in tight containers that maintain a constant, adequate relative humidity. Capsules supplied by the manufacturer in paper boxes should be transferred to amber glass powder squares or other tight containers.
5. Hard-shell capsules consist of two telescoping pieces, a body piece and a cap. In compounding, the two pieces are separated, the body piece is filled with the formulated powder, and then the cap is replaced on the body.

PARTS OF A CAPSULE SHELL
6. Selecting a capsule size for encapsulating a compounded powder
a. Calculate the weight of the powder to be filled into each capsule. This will include the weight of all active ingredients per capsule plus the weight of any needed diluents.
b. A diluent may be needed in the following situations.
(1) If the amount of powder for each capsule is less than the minimum weighable quantity (MWQ) for the balance being used, an inert solid diluent must be added to the active powder ingredients to give a desirable weight per capsule.
(2) Because the smallest capsule sizes, numbers 4 and 5, are difficult for some compounders to handle, if the quantity of powder is more than the MWQ but is so small that a size 4 or 5 capsule shell is needed, diluent may be added so that a larger capsule size can be used. This is demonstrated with the alternate procedure used for Sample Prescription 26.1 on the CD that accompanies this book.
(3) Occasionally, the amount of powder per dose does not fit properly in any given capsule size; it is too much powder to fit in one size but gives void space in the next larger size. If this occurs, an inert solid diluent may be added to adjust the powder volume to the larger-size capsule. This is illustrated with Sample Prescription 36.3 in Chapter 36.
(4) When hand filling capsules, a well-packing diluent may be added to a crystalline or granular powder to improve its ease of packing. Lactose monohydrate (the non-spray-dried variety) and cornstarch are two diluents that work well for this purpose.
(5) When using a capsule-filling machine, an inert diluent may be needed to improve the flow of the powder so that capsules of uniform weight are obtained. Also, the capsule-filling machine may not have plates for all capsule sizes, so you may be required to add diluent to accommodate the capsules sizes available to you.
c. Consult a capsule capacity table such as Table 26.1.
(1) If you have a single capsule ingredient and that ingredient is a representative substance in the table, select the capsule size directly from the table.
(2) Because most formulations are mixtures, selection of a capsule size usually requires judgment and sometimes trial and error. Try to pick a representative substance in the table that has a density similar to that of the ingredient in your formulation that is present in the greatest quantity. If you do not know this information, pick a capsule size that best fits your powder weight for the greatest number of representative drugs and chemicals.
(3) In selecting a capsule size, you want the smallest size that will produce a filled capsule with no void space. For this reason, if your ingredient weight falls between two weights on the table, try the smaller capsule size first.
d. Selecting a capsule size is something of a compromise. For best bioavailability of the powder, a loosely packed capsule is preferred because the powder disperses easily as the capsule shell dissolves. However, when filling capsules individually by hand, it is easier to fill capsules that are slightly packed because, as you fill a number of capsules to a given weight, your fingers will be able to sense the packing pressure that corresponds to the desired weight of capsule ingredients. This makes it easier to achieve the appropriate capsule weight with a minimum number of balance checks. You do not have this sense of pressure with loosely packed capsules.
e. If the amount of powder per unit necessitates a capsule size that is too large for the patient to swallow comfortably, divide the powder per dose in half and put each dose in two capsules. The number of capsules dispensed is then doubled and the directions for use changed to double the number of units administered. The prescriber should be advised of any changes made in the prescription order.
f. The size and color of capsules used for compounding a preparation should be written on the prescription order or included on the compounding record; this will ensure that any refills are prepared with the same capsule size and type. When this information is recorded on the front of the prescription order, the number of the capsule size is usually written inside a triangle, Δ, and the capsule color is recorded beneath the triangle. This is demonstrated for Sample Prescription 26.1 on the CD that accompanies this book.

7. Procedure for hand filling capsules with dry ingredients
a. Prepare the powder using the techniques and procedures for particle size reduction and blending as described in section V of Chapter 25. Make enough powder for one extra capsule, because there will be some loss in the blending and capsule-filling process. If the prescription contains a controlled substance, this loss in compounding must be minimal and should be documented on the prescription order or compounding record.
b. Place the powder mixture for all the capsules on an ointment slab or pad. Using a spatula, arrange the powder into a compact, flat powder bed of uniform thickness. This is sometimes referred to as “blocking the powder bed.” The height of the powder bed should be just slightly shorter than the long dimension of the body piece of the capsule shell. This allows for the most efficient punching of powder into the shell. If the powder bed is even and uniformly packed, it is possible, after punching a few capsules, to get an idea of the number of times to punch powder into each shell body to give the approximate desired weight of powder per capsule.
c. At one time, it was considered permissible to handle capsules with clean hands, but now it is standard procedure to use disposable gloves. In addition to being more sanitary, the use of gloves protects the compounder from contact exposure to the drugs or chemicals being encapsulated. Furthermore, use of gloves eliminates the problem of fingerprints on capsule shells; any dampness on bare fingers will cause a partial dissolution of the gelatin shell and a smudging of its surface.
d. Separate the capsule cap from the body of the capsule shell and repeatedly press the open end of the body of the shell downward into the powder bed. This process is called punching capsules. It is illustrated on Color Plate 2 and is demonstrated using Sample Prescription 26.1 on the CD that accompanies this book.
e. Replace the cap on the body loosely and check the weight of the capsule. The weighing procedure for a double-pan torsion balance is given in Figure 26.1 and, for an electronic balance, in Figure 26.2. Add to or empty powder from the capsule shell until the desired weight is achieved. A tolerance in final capsule weight of ±5% usually can be achieved without too much difficulty.
8. Use of capsule-filling machines
a. Pharmacists who compound capsules routinely or in larger quantities may want to invest in a capsule filler or capsule-filling machine. The nonautomated fillers are available through pharmacy vendors in prices ranging from approximately $20 to more than $3,000. There are also motorized capsule-loading machines in the $5,000 to $10,000 price range that can fill 300 capsules per batch. These fillers work on a principle of calibrated volume fill rather than weight, and their use requires good quality control procedures to ensure precise and accurate dose per capsule.
b. When using a capsule-filling machine, the powder must be formulated so that its flow properties give capsules of uniform weight. Because an inert diluent is usually added to the powder when making capsules by volume, this ingredient should be selected carefully with flow properties in mind.
FIGURE 26.1. PROCEDURE FOR WEIGHING CAPSULES WITH A DOUBLE-PAN TORSION BALANCE
A CAPSULE-FILLING MACHINE
(1) Lactose is a common inert diluent. It is commonly available as the monohydrate, and some suppliers sell the monohydrate in two forms, regular and spray-dried. The regular lactose monohydrate packs well, but it does not have particularly good flow properties. Spray-dried lactose monohydrate has been modified through processing to give a powder with improved flow characteristics. Anhydrous lactose is also available, and it too has good flow properties. Spray-dried lactose monohydrate and anhydrous lactose are good diluents for capsule-filling machines. Check the type carefully when purchasing lactose for use as a diluent in capsule machines.
(2) Microcrystalline cellulose has been used successfully as a diluent for capsule-filling machines. It is a free-flowing powder classified by the NF as a tablet disintegrant and tablet and capsule diluent.
(3) Some granular materials, such as sodium carboxymethylcellulose (CMC), have good flow properties but, depending on the other ingredients in the formulation, they may change the dissolution and absorption characteristics of the drug. Unless specifically called for, natural and synthetic gum powders should be avoided as diluents, because they may form thick viscous barriers around the drug particles when exposed to the aqueous fluid in the stomach and may prevent the drug from being released from the dosage form. Some sustained-release capsule formulations use synthetic gums to modify drug release, but these formulations should always be thoroughly tested before use.
FIGURE 26.2. PROCEDURE FOR WEIGHING CAPSULES WITH AN ELECTRONIC BALANCE
(4) If flow is a problem, one recommendation is to add a small quantity (less than 1% of the total powder weight) of magnesium stearate, a powdered excipient used as a lubricant in manufacturing tablets (6).
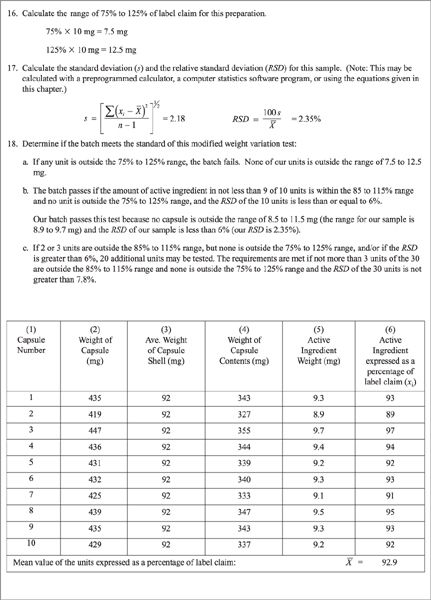
c. As with capsules punched individually, capsules made using a filling machine should always be checked for accuracy and uniformity. A modification of the weight variation test given in Chapter 〈905〉 of USP 23 (7) is useful when evaluating capsules made in this way.
(1) Select 30 capsules and weigh 10 of them individually.
(2) Calculate a mean, a standard deviation, and a relative standard deviation for this sample using these equations:
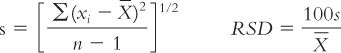
where:

(3) The capsules are satisfactory if all 10 units are within the range of 85% to 115% of the labeled amount of drug per capsule and if the relative standard deviation is less than or equal to 6%.
(4) If this sample of 10 capsules fails to meet these standards, check the 20 additional capsules originally selected; the specifications are met if none of the 30 capsules is outside 75% to 125% and not more than 3 of the 30 is outside 85% to 115% of label claim and the RSD of the 30 capsules does not exceed 7.8% (7).
(5) A quality control record with sample data that utilizes this modified weight variation test is given in Figure 26.3.
d. The modified weight variation test just described checks only for uniformity of fill of the capsules. It assumes that the compounded powder contains the correct amount of active ingredient(s) and that the capsule components are uniformly distributed in the powder mass. To verify that individual capsules contain the labeled amount of drug, pharmacies that routinely compound batches of a given formulation should initially and periodically send batches of the compounded capsules to an analytical lab for testing. If capsules are found to be outside the official USP 〈905〉 content uniformity standard, corrective action should be taken so that compounded capsules are within acceptable limits.
e. An additional double-check procedure that is useful when doing batch compounding is to weigh the containers of formulation ingredients before and after the compounding procedure. The difference in the weight of each container should coincide with the calculated amount of that ingredient in the batch.
f. Pharmacists who compound batches of capsules routinely should also develop written formula and batch record sheets and written standard operating procedures (SOPs). Sample SOPs for capsule making have been published in the International Journal of Pharmaceutical Compounding.
9. Use of manufactured tablets and capsules in compounded hard-shell capsules
a. Manufactured tablets or capsules may be used as ingredients for compounded capsules. Review the discussion on using manufactured dosage forms as compounding ingredients in section II.c. of Chapter 13.
b. Depending on the situation, one of the following techniques may be used:
(1) The tablets may be crushed or the manufactured capsule contents emptied and the resulting powder used as would be any powdered ingredient.
(2) A whole tablet or capsule may be embedded in powder that has been punched into a capsule shell. If the quantity of an ingredient per capsule is the exact quantity in a manufactured tablet or capsule, it may be easier to punch the correct amount of the other ingredients into the shell and then embed the manufactured tablet or capsule in this powder. This technique is particularly useful for protecting ingredients from each other when there is a question about compatibility. This method can also be used when encapsulating tablets or capsules for blind studies. In this case, the capsule shell is partially filled with lactose or another inert diluent, and the tablet or capsule containing the active ingredient is concealed by embedding it in the diluent. If a tablet is concealed in this way, it may be split in two so that a smaller capsule shell can be used. This is especially important when making capsules for children and the elderly, who may have difficulty swallowing larger capsules. This technique is illustrated on Color Plate 3 and is used in Sample Prescription 26.2.
FIGURE 26.3. QUALITY CONTROL RECORD FOR HARD-SHELL CAPSULES
10. Procedure for compounding capsules with liquid ingredients
a. Liquid drugs or solutions or dispersions of drugs may be filled into capsule shells if the shell material is not soluble in the liquid.
b. Care must be exercised to ensure that the liquid does not leak out of the capsule during storage or use.
(1) Leakage can be minimized by sealing the capsule cap to the body of the capsule shell by moistening the inside edge of the cap before replacing it on the body. This can be done with a cotton applicator that has been dipped in water or a water/alcohol solution.
(2) Several brands of capsule shells now are grooved so that the cap snaps in place on the body piece of the capsule. If possible, use capsule shells of this type to prevent leakage of liquid ingredients.
(3) A third method is to mix the liquid drug with a melted miscible material that is a solid at room temperature but will melt at body temperature or will dissolve in the aqueous fluid in the stomach. The solution of the drug in this melted material is added in liquid form to the capsule shell. Because the material solidifies at room temperature, it will congeal and will not leak out of the capsule shell during storage or use. Examples of such drug vehicles are the fatty bases and appropriate blends of polyethylene glycol polymers.
c. Capsule shells may be filled with liquids volumetrically using a syringe or a graduated dropper or pipette.
d. Liquids can also be filled in capsule shells by weight.
FILLING CAPSULES WITH LIQUID INGREDIENTS
(1) Insert the bodies of capsule shells in a holder. (The holder may be as simple as the lid of a powder box with holes punched that are the size of the capsule bodies.)
(2) Place the holder containing the capsule bodies on an electronic balance and tare out the weight.
(3) Using a syringe, dropper, or pipette, add liquid to each shell body to the predetermined desired weight. This procedure is illustrated with Sample Prescription 26.4.
C. Soft-shell capsules or soft-gels
1. The principal material for the shells of these capsules is usually gelatin but, as their name implies, the shell is a softer, more pliable material than that used for hard-shell capsules. This is caused by the presence of glycerin and/or sorbitol, each of which acts as a plasticizer. The shells of soft-gels are also thicker than those of hard-shell capsules (1).
2. These capsules are usually filled with liquids; either the physical form of the active ingredient is a liquid, or a solid active ingredient is dissolved or suspended in a liquid vehicle.
3. The liquid vehicle used in soft-shell capsules must be approved for oral use. It is usually a vegetable oil or a nonaqueous, water-miscible liquid glycol, such as polyethylene glycol 400 or one of the other liquid polyethylene glycols (1).
4. The technology and equipment required for making soft-shell capsules is generally not available in pharmacies, so this dosage form is not usually compounded extemporaneously.
5. Manufactured soft-shell capsules are sometimes used as ingredient sources for compounding.
a. Capsule contents can be extracted either by using a 16- to 20-gauge needle and a syringe to withdraw the liquid contents or by making a slit in the capsule shell and squeezing the contents into the compounding container or measuring device. (See the picture, Extracting surfactant docusate Na from a soft-shell capsule, in Chapter 28.)
b. If the capsule contents are oleaginous, a compatible oil may be added and the resulting oil solution can be used directly, or an emulsion can be made with the addition of water and an emulsifying agent.
c. If the capsule contents have a water-miscible base, such as a liquid polyethylene glycol, they may be added directly to an aqueous or water-miscible compounding vehicle.
III. LOZENGES
A. Uses
1. Lozenges have traditionally been used for local effect—to administer topical anesthetics and demulcents for soothing irritated throat passages experienced with cough and sore throat, and to deliver antibacterial agents intended to promote healing of inflamed or abraded mouth and throat tissues.
2. More recently, lozenges are being used as a way to deliver drugs systemically. As the lozenge slowly dissolves in the mouth, drug is released for absorption in the mouth, either buccally or sublingually, and drug that is swallowed can be absorbed in the gastrointestinal tract.
3. Lozenges are especially useful for patients who have difficulty swallowing oral solid dosage forms. This includes some pediatric and geriatric patients and patients with gastrointestinal blockage.
4. Because lozenges dissolve slowly in the mouth, this dosage form is also useful for medications that give maximum benefit when in prolonged contact with local tissues. Examples include antifungal agents used for the treatment of candidiasis (thrush) and sodium fluoride used for the prevention of dental caries.
5. To enhance patient compliance, especially in children, lozenges are formulated to taste good. Because they may look and taste like candy, lozenges are a potential danger to children; households with children should be warned to keep these preparations out of the reach of children.
B. Types of lozenges
1. Hard lozenges
a. Hard-candy lozenges are mixtures of sucrose and other sugars and/or carbohydrates in an amorphous state. Although they are made from aqueous syrups, the water, which is initially present, evaporates as the syrup is boiled during processing so that the moisture content in the finished product is 0.5% to 1.5% (5).
b. Because making hard lozenges is similar to candy making, helpful hints can be found by consulting a comprehensive cookbook or a candy-making reference. Flavorings, colors, and special molds can be purchased from some vendors of compounding supplies and from businesses that specialize in selling supplies for making candies and confectionaries. Hard-candy lollipops have become an especially popular compounded dosage form in recent years, and special molds and sucker sticks and wrappers are available from various vendors.
c. Successful preparation of smooth hard lozenges depends on careful handling of the syrup and monitoring of temperatures. This is because the crystal-amorphous form of the sugar in the final preparation is temperature and condition dependent. If a formula states that the syrup should not be stirred until a particular temperature is reached, or if it states that the temperature of the syrup must reach 154°C, it is wise to follow these instructions precisely.
d. The finished hardness of the candy depends partly on its water content. To obtain lozenges that are hard and not tacky, the temperature of the melt must reach 149° to 154°C (300° to 310°F). This is called the hard crack stage. This temperature is based on conditions at sea level and average relative humidity. At higher altitudes, these temperatures are lower; on humid days, the syrup should be heated to 1°C higher than usual.
SAMPLE LOZENGE MOLDS
MOLDED HARD-CANDY LOLLIPOP
e. Because of the high temperatures needed to make hard lozenges, drugs or chemicals that are unstable at elevated temperatures should not be incorporated into this dosage form.
f. Formulas for two hard-lozenge bases are given in Table 26.3.
2. Soft lozenges
Soft lozenges can be made with a flavored fatty base (such as chocolate), a polyethylene glycol (PEG) base, or a sugar-acacia base.
a. Fatty-base soft lozenges
(1) The chocolate lozenge base shown in Table 26.3 contains dipping chocolate melted together with a vegetable oil. These lozenges are easy to make and taste good.
(a) The oil depresses the congealing point of the base to facilitate the homogeneous incorporation of active ingredients and the pouring of accurately measured doses.
(b) After melting and mixing, this base can be either poured directly or drawn into a syringe and carefully squirted into tared mold cavities, all without congealing. Plastic medication cups work well as extemporaneous molds.
(c) The finished lozenges should be placed in a freezer to harden for ease of removal from the mold cavities. The removed lozenges should be placed in individual polyethylene bags and stored in the freezer. Because of the presence of oil, these lozenges are too soft to be stored at room temperature. Sample Prescription 26.5 illustrates a compounding procedure for chocolate lozenges.
(2) Table 26.3 also shows a formula for a fatty-base soft lozenge made with a synthetic cocoa butter base and artificial flavor and sweetener. These have a less appealing taste than the chocolate base. Perhaps the chocolate flavor is a needed complement for the oily feel of such a base.
b. PEG-base soft lozenges
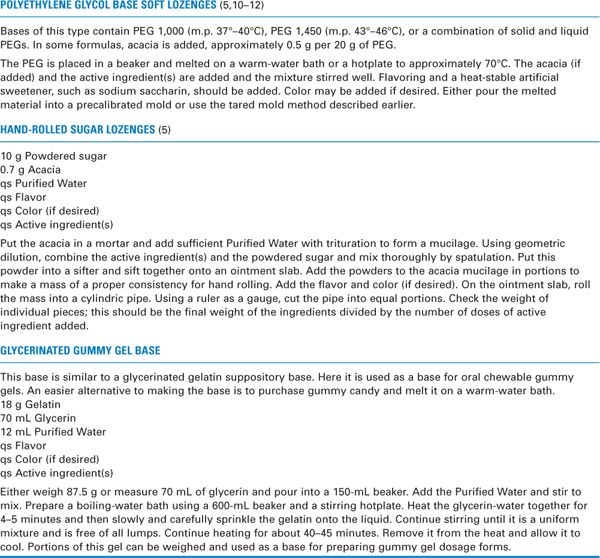
(1) These bases are similar to PEG suppository bases except that they are formulated to be less firm. Most commonly, PEG 1,000, with a melting point of 37° to 40°C, or PEG 1,450, with a melting point of 43° to 46°C, is used alone or with added acacia, approximately 0.5 g per 20 g of PEG base. Some formulas are more complex and use a combination of solid and liquid PEGs to give a particular desired consistency. (10–12)
(2) To make these soft lozenges palatable, flavoring and a heat-stable artificial sweetener, such as sodium saccharin, should be added. Color may be added if desired. A sample compounding procedure is given in Table 26.3. Even with added flavor and sweetener, these lozenges would not be considered tasty. If this sort of base is used, considerable experimentation with flavoring and sweetener must be done to formulate a preparation with a satisfactory taste.
c. Hand-rolled acacia-sugar lozenges
(1) The base material for this lozenge is powdered sugar held together by an acacia mucilage.
(2) These lozenges are among the simplest to make. A sample formula and a procedure are given in Table 26.3 and are illustrated in Sample Prescription 26.7. The general compounding procedure is given later in this section (see III.C.2).
3. Chewable gummy gel lozenges
a. This lozenge base is similar to the old-fashioned glycerinated gelatin that was used for many years as a base for vaginal suppositories. It made its appearance as a base for chewable oral drug preparations after a candy for children, so-called gummy worms or gummy bears, was introduced and became popular.
b. A gummy gel base, such as that shown in Table 26.3, can be made from scratch in the pharmacy. This requires time and patience, because first the material must be heated carefully with stirring for 40 to 45 minutes, and this is only the beginning! To make a palatable product, appropriate flavoring and sweetening must then be added; citric acid is sometimes added to improve the taste—the tartness it provides takes away from the acrid taste of the glycerin. In addition, acacia may be added to provide smoothness. If the active ingredient or ingredients are insoluble in the base, a small amount of a suspending agent, such as bentonite, may also be added (13). In short, making this base is a time-consuming process.
c. An alternative to making the gummy gel base is to purchase gummy candy. It can be heated in a beaker on a warm-water bath until a fluid is obtained. This gives a flavorful base of a desirable consistency.
C. General compounding methods for lozenges
1. Lozenges are similar to suppositories in that they can be made either by hand rolling or by fusion. Depending on the compounding method selected, special calculations, compounding techniques, and equipment may be required to give accurate doses of lozenges.
2. Hand-rolled lozenges
a. Advantages
(1) Hand-rolled lozenges do not require special calculations.
(2) Special equipment is not required for this method. A pill roller is useful, but a broad-bladed spatula or any stiff, flat piece of nonreactive material can be used for this purpose.
b. Disadvantages
(1) Preparing and forming hand-rolled lozenges requires experience and good technique.
(2) Even when well made, hand-rolled lozenges do not have an elegant appearance.
c. General compounding method (used in Sample Prescription 26.7)
(1) Check the doses of the active ingredients.
(2) Using general principles, create a base formula or, if the lozenge base formula is taken from a journal article or book, adapt that formula for your specific use. The desired finished weight per lozenge is usually 1 to 2 g.
(3) Calculate the quantity of each ingredient needed for compounding the preparation. Make enough material for two extra lozenges.
(a) Multiply the dose per unit times the number of units to determine the quantity of each active ingredient.
(b) Multiply the finished weight per unit times the desired number of units.
(c) Subtract the weight of the active ingredients from the total weight of the lozenges to determine the amount of base material.
(4) Weigh and prepare the active ingredients and base materials.
(5) Combine the ingredients to form a cohesive mass.
(6) Place the mass on an ointment slab and roll into a cylindric pipe of an appropriate length.
(7) Using a clean razor blade and a ruler for a gauge, cut the pipe into equal pieces of the desired number of dosage units.
(8) Weigh each piece and shave off extra material if needed to achieve lozenges of the correct weight.
3. Molded lozenges, fusion method (using heat)
a. Advantages
(1) Some of the better-tasting lozenges, such as hard-candy, chocolate, and gummy gel chewable lozenges, can be made only by using heat and molding.
(2) When well made, the preparations have a finished, professional appearance. Special molds, including those to make lollipops, are available from some vendors of compounding supplies and from confectionaries.
b. Disadvantages
(1) Special molds are usually required to make lozenges by molding. It is possible to improvise by using the caps of such items as vials or plastic medication cups for mold forms.
(2) Handling some types of base materials requires special skill, experience, and care to obtain satisfactory preparations.
(3) Caution must be used when incorporating drugs sensitive to heat.
(4) Although the dosage units of molded lozenges may be determined either by weight or by volume, both methods require special equipment, calculations, or procedures.
c. General compounding methods
(1) By weight (illustrated with Sample Prescription 26.5)
(a) When lozenges are compounded to a final weight, an electronic digital balance is nearly a necessity.
(b) Follow steps (1) through (4) as given previously for hand-rolled lozenges.
(c) Using heat, prepare the lozenge base material.
(d) Add the active ingredient(s) to the molten base.
(e) Place the lozenge mold(s) on an electronic balance and tare out the weight of the mold(s).
(f) Pour melted lozenge material into each mold cavity to the calculated desired weight per lozenge using the balance digital readout as the gauge. In some cases, it is easier to first draw the lozenge material into a syringe and use this to add the molten material to the mold cavities.
(2) By volume
(a) If volume is used, density calculations, mold calibrations, double-casting, or other procedures are required to give accurate doses.
(b) Because lozenges are solid at room temperature, most of the base components and active ingredients are solids and are measured by weight. The components are then combined, melted, and poured into mold cavities. This means the quantity of the dosage unit is determined by volume—the volume of the mold cavity.
(c) The quantity of active ingredient in each dosage unit therefore depends on two factors: the w/w concentration of active ingredient in the base material and the weight of the mixture contained in each mold cavity. The weight of mixture in the mold cavity depends on the volume of the cavity and the density of the molten mixture.
(d) To determine the quantity of base and active ingredients(s) to weigh when using this method, it is necessary to either calibrate the mold cavities for the desired material or use a double-casting procedure. Descriptions of these procedures are given in examples 31.1 through 31.3 in Chapter 31, Suppositories.
IV. MOLDED TABLETS
A. Molded tablets, sometimes called tablet triturates
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree