OUTLINE
Mechanism for Auto-Oxidation of Pharmaceutical Compounds
Properties of the Ideal Antioxidant/Chelating Agent
Antioxidants Listed in the USP 30/NF 25
Chelating Agents Listed in the USP 30/NF 25
Antioxidants for Aqueous Systems
I. DEFINITIONS
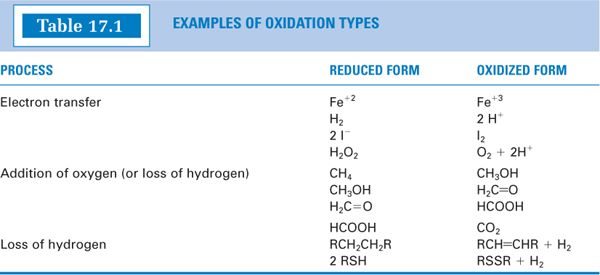
A. Oxidation/reduction (redox) reactions involve the transfer of one or more oxygen or hydrogen atoms or the transfer of electrons (1). (See Table 17.1 for examples.) Writing an electron transfer in equation form, where e− represents an electron and n the number of electrons:
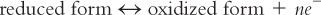
The section on oxidation in USP Chapter 〈1191〉, Stability Considerations in Dispensing Practice, describes and relates oxidation to pharmaceutical preparations as follows:
The molecular structures most likely to oxidize are those with a hydroxyl group directly bonded to an aromatic ring (e.g., phenol derivatives such as catecholamines and morphine), conjugated dienes (e.g., vitamin A and unsaturated free fatty acids), heterocyclic aromatic rings, nitroso and nitrite derivatives, and aldehydes (e.g., flavorings). Products of oxidation usually lack therapeutic activity. Visual identification of oxidation, for example, the change from colorless epinephrine to its amber colored products, may not be visible in some dilutions or to some eyes.
Oxidation is catalyzed by pH values that are higher than optimum, polyvalent heavy metal ions (e.g., copper and iron), and exposure to oxygen and UV illumination. The latter two causes of oxidation justify the use of antioxidant chemicals, nitrogen atmospheres during ampul and vial filling, opaque external packaging, and transparent amber glass or plastic containers (2).
B. Auto-oxidations are oxidations that occur spontaneously under normal conditions of preparation, packaging, and storage.
C. A free radical is a chemical species that has an unshared electron in its outer shell. [Oxygen (O2) has an electronic configuration with two unshared electrons in its outer shell.]
D. Antioxidants are substances that prevent or inhibit oxidation. They are added to dosage forms to protect components of the dosage form that are subject to chemical degradation by oxidation.
E. Chelating agents are organic compounds that can form complexes with metal ions and, in so doing, inactivate the catalytic activity of the metal ions in the oxidation process.
II. PREVENTING OXIDATION
The pharmacist can try the following to prevent or minimize oxidation during compounding (3):
A. Use deaerated water. Boil Purified Water for 5 minutes and immediately cover it so that it does not come into contact with air that may redissolve in it. Although deaerated water is not an official USP article, the USP recognizes this type of water in Chapter 〈1231〉, Water for Pharmaeutical Purposes, and in the Reagents section of USP, where it states that deaerated water is produced by boiling vigorously for 5 minutes and cooling or by applying ultrasonic vibration (4,5).
B. Incorporate the antioxidants in the preparation as early in the process as possible. If a system with multiple phases is made, such as an emulsion, place an antioxidant in each phase as early in the process as possible.
C. Do not use a mixing method or device that incorporates air into the system.
D. Use a mixing container that has minimal headspace and/or replace the air in the headspace with nitrogen.
E. Add a buffer system to maintain a desired pH.
F. Use ingredients with low heavy-metal content.
G. Decrease the temperature during preparation, if possible.
III. USES OF ANTIOXIDANTS
A. Antioxidants and/or chelating agents may be added to manufactured pharmaceutical products and to extemporaneously compounded preparations when the product or preparation contains an ingredient or ingredients—either an active ingredient or a dosage form component—that is subject to chemical degradation by oxidation.
B. For compounded preparations, the decision by the pharmacist whether or not to add an antioxidant or chelating agent is made by taking into consideration the susceptibility of the ingredient(s) to degradation, the dosage form, the targeted site of drug delivery (e.g., topical, oral, ophthalmic, parenteral), the packaging for the preparation, the anticipated conditions of storage and use of the preparation, and the beyond-use date desired or needed.
IV. MECHANISM FOR AUTO-OXIDATION OF PHARMACEUTICAL COMPOUNDS
To appreciate how antioxidants and chelating agents function in preventing or retarding oxidation, it is helpful to have a basic understanding of the oxidation process. Oxidation is complex, and the following is just a brief outline of that process. For an excellent review on the subject of oxidation in pharmaceutical products and preparations, see the chapter on oxidation and photolysis in Chemical Stability of Pharmaceuticals (6).
A. Oxidation reactions
Auto-oxidation of pharmaceutical ingredients occurs by a series of free radical chain reactions, including initiation, propagation, and termination.
1. Initiation

Note: This reaction may be catalyzed by heat, light, and metal ions.
2. Propagation
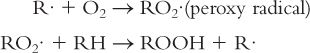
3. Termination

B. To prevent or inhibit oxidation, the stabilizing compound must prevent or interfere with initiation or propagation or it must participate in a termination step.
1. Chelating agents inhibit oxidation by complexing metal ions that act as catalysts for some oxidation reactions.
a. Metal ions, such as Fe+3, Cu+2, Co+3, Ni+2, and Mn+2, can act as initiators of oxidation because they each have an unshared electron in their outer shell.
b. Drug products and preparations may easily be contaminated by trace amounts of metals because these contaminants may be present in minute amounts, even in high-quality compounding ingredients and on the surfaces of compounding equipment and packaging materials.
c. Chelating agents, such as ethylenediaminetetraacetic acid (also known as EDTA or edetic acid), citric acid, and tartaric acid, act by binding the metal ions through complexation. Their binding capacity is pH dependent because it depends on the degree of ionization of these organic acids; they are most effective when fully ionized so they lose their ability to complex at low pH.
2. Antioxidants may function by one of the following mechanisms:
a. Some are compounds that are easily oxidized; they have lower oxidation potentials than the drugs they are intended to protect and are preferentially oxidized. These agents act as so-called oxygen scavengers. Examples include the sulfites, ascorbic acid, monothioglycerol, and sodium formaldehyde sulfoxylate.
b.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree