OUTLINE
I. GENERAL INFORMATION
A. Water is the most commonly used and most desirable solvent-vehicle for liquid drug products and preparations for all uses.
B. Other solvent-vehicles frequently used as ingredients in drug products and compounded preparations include alcohol, isopropyl alcohol, glycerin, propylene glycol, and polyethylene glycol 400.
C. Some other solvents are used pharmaceutically in processing drug products, for assays and tests, or for making specialty products and preparations such as Flexible Collodion. Examples include acetone, ether, and chloroform.
D. Oils used as pharmaceutical solvents-vehicles include a variety of vegetable oils and mineral oil. Examples include corn oil, cottonseed oil, and almond oil. Some special vegetable and essential oils are used primarily as flavors and scents. The National Formulary section of the USP–NF (1) has monographs for various oils of this type, such as anise oil, lemon oil, and rose oil. These are discussed in Chapter 21, Colors, Flavors, Sweeteners, and Scents.
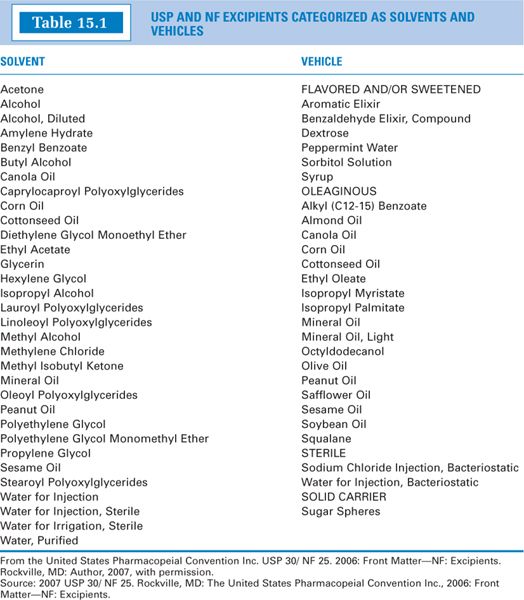
E. The USP–NF lists official articles classified as solvents and vehicles by categories in a table of excipients in the Front Matter of the NF section (2). These are given in Table 15.1. Notice that some articles are listed in both categories and some, such as Sterile Water for Inhalation and Dehydrated Alcohol, are not listed at all. The following chapter describes those articles most frequently encountered in pharmacy compounding and practice.
F. In reading and interpreting the current chapter, note that this text employs the usual convention of using upper-case first letters for words designating official USP–NF articles (e.g., Alcohol, Purified Water) and lower-case first letters for words designating the chemical substances (e.g., ethanol, water).
G. Recently, the USP also started listing some cyclodextrins that are used as solubilizing agents.
II. WATER
A. General information
1. Water is such an important substance and component of pharmaceutical dosage forms that the USP describes and sets standards for various types of water in the General Notices official monograph, and Chapter 〈1231〉 Water for Pharmaceutical Purposes.
2. There are eight official types of water. These are USP Purified Water; USP Water for Injection; USP Water for Hemodialysis; USP Sterile Water for Injection; USP Sterile Water for Inhalation; USP Bacteriostatic Water for Injection; USP Sterile Water for Irrigation; and USP Sterile Purified Water. (3). The USP designation means that the water is the subject of an official monograph in the current USP with various specifications for each type. The latter four waters are “finished” products that are packaged and labeled as such. The USP Purified Water, USP Water for Injection, and USP Water for Hemodialysis conversely, are components or “ingredient materials” as they are termed by the USP, intended to be used in the production of drug products.
3. When water is used in making official USP preparations, it must meet the criteria specified in the USP for the type of preparation being made. For example, the water used for making parenteral products must meet the requirements for injections found in Chapter 〈1〉 Injections of the USP (4).
4. The basic starting ingredient for all USP water items is potable (drinking) water as defined in the General Notices of the USP: “Potable water meeting the requirements for drinking water as set forth in the regulations of the federal Environmental Protection Agency may be used in the preparation of official substances” (1). This means that drinking water may be used in the manufacturing and preparation of USP drug substances, including water articles. Drinking or tap water does not, however, meet the standards as an ingredient in dosage forms. Water for making dosage forms must be one of the official USP monograph water articles as described further.
5. In addition to Water for Injection and Purified Water USP, in Europe there is a third class of water called highly purified water, like a super grade of Purified Water USP. Unlike Water for Injection, which is traditionally produced with a still, highly purified water is not boiled. Instead, it goes through various pretreatment steps, one or two reverse osmosis passes, a deionization step, and sometimes ultraviolet radiation and a final filtration step. The end result is highly purified water that is equivalent to Water for Injection but produced at a lower cost.
B. USP–NF water articles
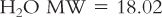
a. Preparation
(1) Made from water complying with the U.S. Environmental Protection Agency (EPA) National Primary Drinking Water Regulations or comparable regulations of the European Union or Japan
(2) Processed by distillation, ion-exchange treatment, reverse osmosis, or other suitable method
(3) No added substances (such as preservatives)
b. Description: clear, colorless, odorless liquid
c. Standards
(1) Meets USP requirements for Total Organic Carbon 〈643〉 and Water Conductivity 〈645〉
(2) Bacterial endotoxins (pyrogens): no standard
(3) Bacteriologic purity: complies with EPA regulations for drinking water
d. Packaging and storage:When packaged, use tight containers.
e. Labeling:When packaged, label method of preparation.
f. Uses
(1) USP Chapter 〈795〉 states that for nonsterile compounding, pharmacists must use USP-grade purified water (6), which must meet criteria (e.g., for dissolved solids, pH) that ensure the stability of preparations.
(2) It is used as a solvent-vehicle for the preparation of pharmaceutical dosage forms for internal or external use.
(3) It is not for use when sterility is required unless it meets the requirements under USP Sterility Tests 〈71〉 or is first sterilized by filtration or autoclaving. It must then be protected from microbial contamination.
(4) It is not for use in making parenteral products unless it can be assured that it meets the requirements for sterility and bacterial endotoxins for parenteral administration.
2. Sterile Purified Water USP (3,5)
a. Preparation
(1) Made from Purified Water that has been sterilized and suitably packaged
(2) No added substances (such as preservatives)
b. Description: clear, colorless, odorless liquid
c. Standards
(1) Meets USP requirements for Total organic carbon 〈642〉 and Water conductivity 〈645〉. Total organic carbon (TOC) is an indirect measure of organic molecules present in pharmaceutical waters measured as carbon. Electrical conductivity in water is a measure of the ion-facilitated electron flow through it and Water conductivity is therefore a measure of the presence of extraneous ions
(2) Bacterial endotoxins (pyrogens): no standard
(3) Bacteriologic purity: meets requirements in Chapter 〈71〉 for Sterility. Portions of this general chapter in the USP have been harmonized with the corresponding texts of the European Pharmacopeia and/or the Japanese Pharmacopeia
(4) pH: between 5.0 and 7.0
(5) Other standards as given in the USP for ammonia, calcium, chloride, sulfate, and oxidizable substances
d. Packaging and storage: suitable tight containers
e. Labeling: Label with method of preparation and that this is not for parenteral use.
f. Uses
(1) It is used as a solvent-vehicle for the preparation of pharmaceutical dosage forms for internal or external use.
(2) It is not for parenteral administration.
3. Water for Injection USP (3,5)
a. Preparation
(1) Purified by distillation or reverse osmosis not by deionization because bacterial endotoxins can grow in a deionizing chamber to a level exceeding USP requirements
(2) No added substances (such as preservatives)
b. Description: clear, colorless, odorless liquid
c. Standards
(1) Bacterial endotoxins (pyrogens, USP Chapter 〈151〉 Pyrogen Test): not more than 0.25 USP Endotoxin Units per mL (USP Chapter 〈85〉 Bacterial Endotoxins Test)
(2) All requirements for Purified Water
d. Uses: Water for Injection is a starting material for making parenteral products. It must be processed further either before use or during product preparation. The USP monograph has the following note on its use:
NOTE—Water for Injection is intended for use as a solvent for the preparation of parenteral solutions. Where used for the preparation of parenteral solutions subject to final sterilization, use suitable means to minimize microbial growth, or first render the Water for Injection sterile and thereafter protect it from microbial contamination. For parenteral solutions that are prepared under aseptic conditions and are not sterilized by appropriate filtration or in the final container, first render the Water for Injection sterile and thereafter, protect it from microbial contamination (5).
4. Sterile Water for Injection USP (3,5)
a. Preparation
(1) Made from Water for Injection that is sterilized and suitably packaged
(2) No added substances (such as preservatives)
b. Description: clear, colorless, odorless liquid
c. Standards
(1) All requirements given for Sterile Purified Water
(2) Particulate matter: Meets requirements in USP Chapter 〈788〉 Particulate Matter in Injections. In this context, particulate matter is defined as mobile, randomly sourced, extraneous substances, other than gas bubbles, that cannot be quantitated by chemical analysis because of the small amount of material present (7). Injectable solutions, including solutions constituted from sterile solids intended for parenteral use, must be free from particulate matter that can be observed on visual inspection (4,7).
(3) Bacterial endotoxins (pyrogens): not more than 0.25 USP Endotoxin Units per mL
(4) Bacteriologic purity: meets requirements given for Sterile Purified Water (Chapter 〈71〉 Sterility Tests)
d. Packaging and storage: single-dose glass or plastic containers not larger than 1 liter. Glass containers of Type I or Type II glass are preferred.
e. Labeling: Label to indicate that no preservative or other substance has been added. Also label that it is not suitable for intravascular injection unless it is first made approximately isotonic by the addition of a suitable solute.
f. Uses
(1) Base vehicle for large-volume parenteral fluids
(2) Solvent for drugs intended for parenteral use
5. Sterile Water for Inhalation USP (3,5)
a. Preparation
(1) Made using Water for Injection that is sterilized and suitably packaged
(2) No added substances, except that antimicrobial agents may be added when the water is to be used in humidifiers or other devices in which it may become contaminated
b. Description: clear, colorless solution
c. Standards
(1) All requirements given for Sterile Purified Water except pH
(2) Bacterial endotoxins (pyrogens): not more than 0.5 USP Endotoxin Units per mL
(3) Bacteriologic purity: meets USP sterility requirements
(4) pH: 4.5 to 7.5
d. Packaging and storage:Glass or plastic containers;Type I and II glass preferred for glass containers
e. Labeling: Label that it is for inhalation therapy only and not for parenteral use.
f. Uses
(1) In humidifiers to add moisture to the environment
(2) As a solvent for drugs to be administered by inhalation
(3) The following is a note on use in the USP monograph:
NOTE—“Do not use Sterile Water for Inhalation for parenteral administration or for other sterile compendial dosage forms” (5).
6. Sterile Water for Irrigation USP (3,5)
a. Preparation
(1) Made from Water for Injection that is sterilized and suitably packaged
(2) No added substances (such as preservatives)
b. Description: clear, colorless, odorless liquid
c. Standards
(1) All requirements given for Sterile Purified Water
(2) Bacterial endotoxins (pyrogens): meets the endotoxin test under Water for Injection
(3) Bacteriologic purity: meets requirements given for Sterile Purified Water
d. Packaging and storage: single-dose glass or plastic containers. Glass containers of Type I or Type II glass are preferred. Containers may have volumes in excess of 1 liter and may be designed with a closure to facilitate easy, rapid emptying.
e. Labeling: Label to indicate that no preservative or other substance has been added. Also, the labels “For irrigation only” and “Not for injection” must be conspicuous.
f. Uses
(1) Irrigation fluid
(2) Solvent for drugs to be administered by irrigation, usually for local effect
7. Bacteriostatic Water for Injection USP (3,5)
a. Preparation
(1) Made from Sterile Water for Injection that has been sterilized and suitably packaged
(2) Added substances: one or more suitable antimicrobial agents are added
b. Description: clear, colorless liquid, odorless, or possibly having the odor of the added antimicrobial agent(s)
c. Standards
(1) Particulate matter: meets requirements for Sterile Water for Injection
(2) Bacterial endotoxins (pyrogens): not more than 0.5 USP Endotoxin Units per mL
(3) Bacteriologic purity: meets USP sterility requirements
(4) pH: 4.5 to 7.0
(5) Meets the effectiveness requirements of Chapter 〈51〉 Antimicrobial Effectiveness Testing and label claim for content of the antimicrobial agent(s) in Chapter 〈341〉 Antimicrobial Agents–Contain
(6) Meets all requirements for Sterile Purified Water except pH, ammonia, chloride, and oxidizable substances
d.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree