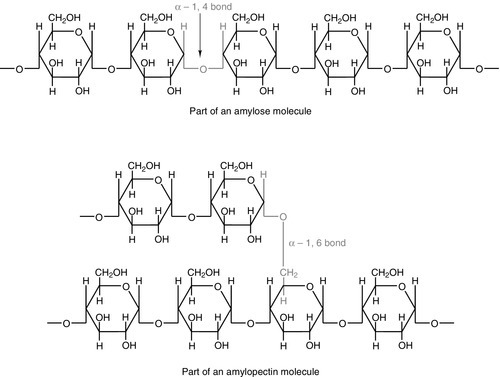
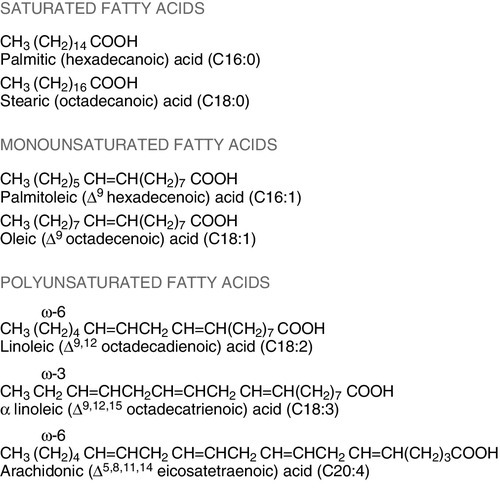
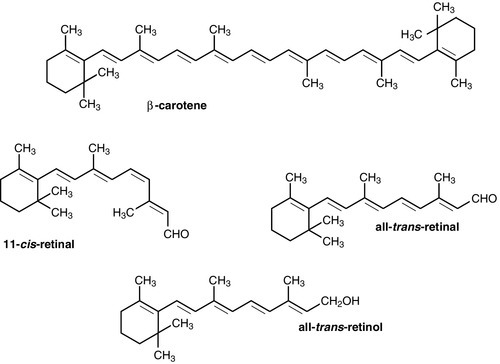
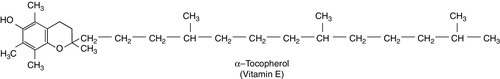
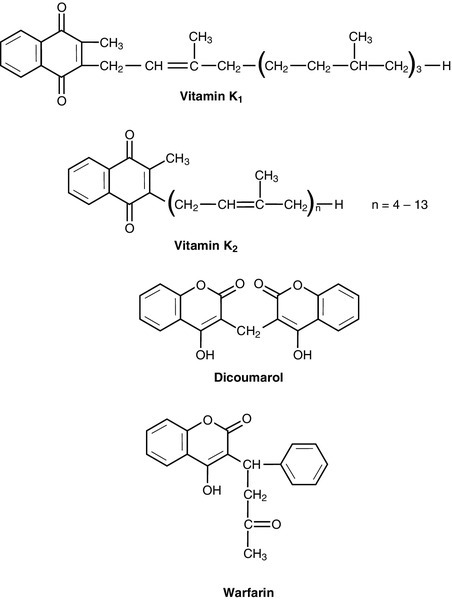
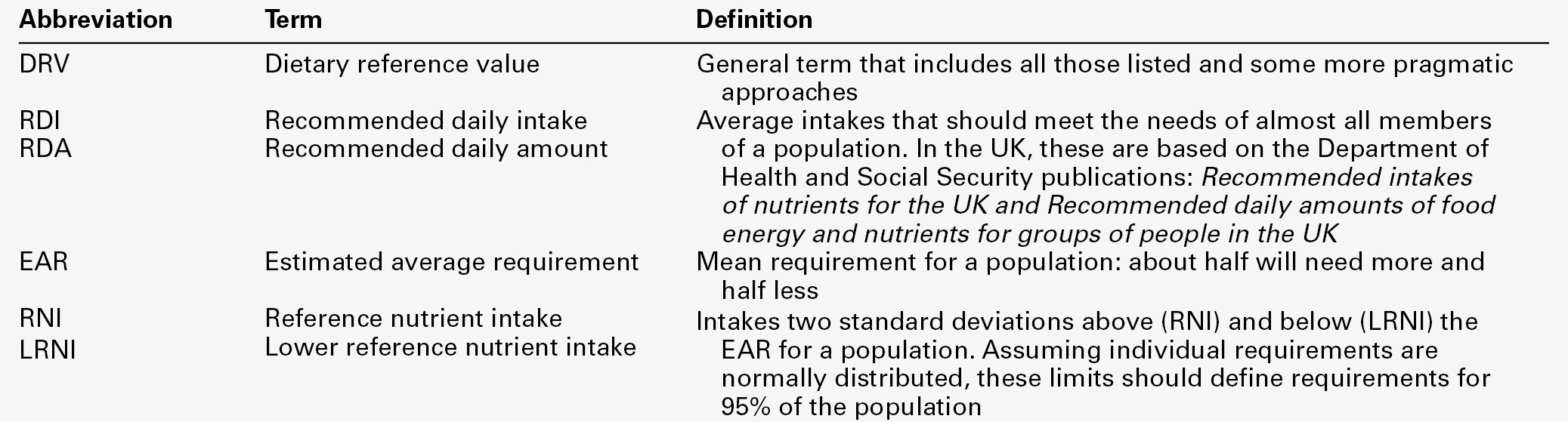
CHAPTER 10 CHAPTER OUTLINE Food is essential for human life. At the extremes, too little leads to starvation and too much leads to obesity, each with its associated effects on morbidity and mortality. Between these extremes, different people across the world follow apparently very different diets with no obvious resulting differences in their day-to-day health. It is apparent that in the long term the composition of the diet has an effect on the incidence of certain diseases, for example ischaemic heart disease and certain types of cancer, but often these associations are difficult to tease out because of the many confounding factors involved. However, this does not prevent very firm opinions being formulated and held about what is and what is not ‘good nutrition’, based on a variety of influences from basic science to religion. Biochemistry clearly has a role in establishing the way in which the body uses various nutrients and has been important in defining certain deficiency states. Clinical biochemistry is still important in diagnosing deficiencies of certain specific nutrients, but whether there is a good biochemical marker of overall nutritional status is less clear. This chapter begins with a consideration of the various nutrients, including the effects of their deficiency or excess, and then discusses nutritional assessment. The following chapter discusses nutritional disorders and their management, both from the point of view of nutrition as an aetiological factor in disease, and disorders that are not primarily nutritional but where dietary modification or nutritional support may be important in treatment. Part of the explanation of the fact that apparently very different diets can sustain life equally well is the concept that individual foodstuffs actually comprise different combinations of certain basic nutrients, and that it is the supply of these basic nutrients that is important rather than their origin. However, even when individual nutrients are considered, the definition of the ‘correct’ intake is problematical since this may be taken, for example, to be any of the following: the intake that avoids clinical signs of deficiency; the intake that maintains a given circulating concentration or tissue content of the nutrient; the intake that cures symptoms or signs of clinical deficiency; the intake that maintains a balance between intake and consumption or loss from the body over a defined period, or any one of a variety of other definitions. Even if a suitable measure can be determined for a specific nutrient, there are further problems in setting the absolute figure, since within a population, requirements will vary between individuals (owing, for example to differences in age, gender size and body composition) and even in an individual, requirements may vary in the short term (owing, for example to pregnancy, illness or environmental stress). It is thus difficult to accurately estimate average requirements for a nutrient, and most attempts to make recommendations about intakes have depended on setting levels where most of the population will not be deficient, although it is also possible to define intakes below which most of a population will develop deficiency, and in some cases intakes that are high enough to be toxic. These points are covered in more detail in some of the texts listed under Further reading, below, and no attempt will be made here to define ideal intakes in absolute terms. Some of the terms used to describe nutrient requirements are defined in Table 10.1. TABLE 10.1 Definitions of terms used in describing nutrient requirements Adapted from Department of Health 1991. The body uses energy in the maintenance of metabolic processes, in physical activity and in growth. In the resting individual, energy-requiring processes include the active pumping of ions across cell membranes, thermoregulation, cell division and basal function of all of the body’s systems. Resting energy expenditure appears to alter with energy intake to some extent. Physical activity, particularly if vigorous, can increase energy expenditure markedly, although such activity is not usually sustained for long enough to make the increase substantial when measured over a 24 h period. The laying down of new tissues represents an investment of energy, so it is not surprising that energy requirements, expressed in terms of body weight, are highest in infants and young children, with a gradual decline from the third decade into old age. Thus, the energy requirement of an individual varies with body size and composition, gender, age, nutritional status and climate. In women, energy expenditure is increased during pregnancy and lactation, and in any individual, illness or response to trauma (e.g. systemic infection, burns) may cause a considerable increase. Energy expenditure and energy intake do not always rise and fall in parallel. A sustained deficiency in energy intake generally leads to consumption of body stores of energy, including protein as well as glycogen and fat. Excessive energy intake (if sustained) results in obesity. Both of these conditions are considered further in the next chapter. Dietary energy is mainly obtained from a combination of fat and carbohydrate. In a Western diet, each may account for 40–50% of total energy intake, although in developing countries the proportion from carbohydrate tends to be higher and that from fat, lower. Although dietary protein is generally thought of as a source of materials (amino acids) for endogenous protein production, it may also be used for energy production, for example if protein intake is adequate but non-protein energy intake is low. Ethanol also has to be considered as a significant energy source in certain populations; for example, in the UK, it forms 7% of average energy intake. (Interestingly, at higher ethanol intakes – 25–35% of dietary energy – ethanol appears not to be completely utilized as a source of energy, although the reason for this is not clear.) Dietary carbohydrate provides 4.1 kcal/g and most is derived from sugars and starch. The sugars are mainly the monosaccharides glucose, fructose and galactose, and the disaccharides sucrose, lactose and maltose. Dietary sugars can be divided into those which are present in intact cells, such as in whole fruit (intrinsic sugars), and those which are free and readily absorbed as a result of having been added to food, usually as sucrose (extrinsic sugars). Milk sugars are usually considered as intrinsic. A high intake of extrinsic sugars is associated with an increased prevalence of dental caries. Sugar derivatives, such as the sugar alcohols sorbitol and xylitol, can be partially digested and can provide 2.4 kcal/g. In the UK, it is recommended that in the adult, 40–50% of energy intake should be provided by carbohydrate, but non-milk extrinsic sugars should not exceed 11% of energy intake. There is considerable debate about whether a high intake of non-milk extrinsic sugars may have harmful effects in addition to the increased risk of dental caries. If such an intake results in an increased energy intake, then obesity is likely to be a problem, but in any case, ingestion of large amounts of extrinsic sugars can lead to raised plasma glucose, insulin and lipid concentrations, all of which are potentially harmful. Large amounts of starch are not harmful: indeed, in developing countries, starch may form 75–80% of the total energy intake. Starches are α-glucan polysaccharides, of which there are two major forms, amylose and amylopectin (see Fig. 10.1). They are partially resistant to hydrolysis during digestion and are thus not totally available as an energy source. Certain other carbohydrates, that are not energy sources, but form part of ‘dietary fibre’, are considered later. A deficiency in dietary carbohydrate will either lead to energy deficiency or result in potentially harmful amounts of other energy sources being required in the diet if energy intake is to be maintained. However, certain tissues have an obligatory requirement for glucose as an energy substrate (e.g. brain and nervous system (in the short term), red blood cells) and, while this need can be met by gluconeogenesis, some carbohydrate is necessary in the diet if ketosis is to be avoided. The term ‘fat’ includes a range of substances, including triacylglycerols (triglycerides), phospholipids and sterols (e.g. cholesterol). Triacylglycerols are the most common storage fats and are thus the most important fatty energy source in foods. They are a relatively ‘concentrated’ energy source, with a higher energy content per unit weight (9.3 kcal/g) than carbohydrate (4.1 kcal/g). Although all triacylglycerols can act in this way, there may be important long-term health differences between them, dependent on the structure of the fatty acid residues they contain. The main differences are in the length of the carbon chain, the presence (and number) of any unsaturated bonds and, if such bonds are present, the positional and geometric isomers present. Some examples of these differences are illustrated in Figure 10.2. The effects of excessive intake of triglycerides are discussed further in Chapter 11, and the differences in absorption and metabolism between long chain and medium chain triglycerides in Chapter 12. Dietary fat deficiency does not generally seem to be a problem, as most fats necessary to the body can be endogenously synthesized when a high proportion of total energy intake is supplied by carbohydrate. However, there are certain fatty acids that appear to be essential, at least in small amounts. These are linoleic acid (C18:2, ω-6) (for nomenclature, see Fig. 10.2) and α-linolenic acid (C18:3, ω-3). They are important components of phospholipids, in which they help to maintain the function of cellular and subcellular membranes. They are also involved in the regulation of cholesterol transport, breakdown and excretion. They are the precursors of arachidonic (C20:4, ω-6), eicosapentaenoic (C20:5, ω-3) and docosahexaenoic (C22:6, ω-3) acids and are thus also important in the synthesis of prostaglandins, thromboxanes and leukotrienes. These three longer chain fatty acids are not strictly essential fatty acids (EFAs), but may become conditionally so in EFA deficiency. Adequate dietary supply of the longer chain fatty acids is probably also important during rapid brain growth in infancy, particularly in fast-growing premature infants. The clinical effects of EFA deficiency include dermatitis, alopecia and fatty liver, but these are only seen when EFAs provide less than 1–2% of total dietary energy intake. In patients receiving long-term fat-free parenteral nutrition, cutaneous application of small amounts of appropriate oils has alleviated biochemical EFA deficiency. Proteins have a key structural and functional role in virtually all bodily processes, and a supply of appropriate amino acids is necessary during normal turnover, with additional amounts if extra protein is being formed, for example during growth, pregnancy or lactation. This supply generally comes from dietary protein, of either animal or vegetable origin, but the actual amount of protein required depends on its amino acid content. Human protein contains 20 amino acids (Box 10.1), of which nine are recognized to be essential (or ‘indispensable’) in adults – the remaining 11 can be synthesized endogenously if the food protein is deficient in these but contains sufficient essential amino acids. Some amino acids, for example cysteine and glutamine, whilst not normally essential, may become so in conditions such as severe illness, trauma or burns, when requirements exceed the body’s ability to synthesize them. In children, due to the increased demands of growth, the threshold for an amino acid to become conditionally essential is lower. Only L-amino acids are incorporated into human proteins. Some proteins, particularly those derived from animal sources such as eggs, milk, fish and meat, contain all the essential amino acids in sufficient amounts for protein synthesis and are said to be complete, or of high quality. Foods lacking one or more amino acids are termed incomplete proteins; these are often from plant sources such as pulses. In diets in which plant foods are the main source of protein, combining different types of food in appropriate quantities is required in order to obtain all the essential amino acids. However, the amino acid profile of dietary proteins does not completely predict the amounts of these amino acids that will be available for protein synthesis after digestion and absorption: some of them may be biologically unavailable. Lysine is particularly important in this regard, as its supply in many dietary proteins is relatively low in comparison to nutritional requirements. It becomes unavailable if its ε-amino group reacts with another molecule, because ε-amino bonds are not cleaved by digestive enzymes. Such bonds may form with other amino acids (e.g. the carboxyl side chains of glutamic or aspartic acids) or with reducing sugars (during cooking or storage of foods). Deficiency of protein generally occurs as part of more generalized malnutrition and is discussed later. Excessive protein intake in renal disease accelerates deterioration in renal function (see Chapter 7) and it is possible that it may have effects in healthy people, for example on renal function and on bone mineral density. Vitamins and certain trace elements are essential components of the diet required in very small amounts. Deficiency of individual micronutrients classically results in typical symptoms and signs according to the vitamin or trace element involved; recognition and treatment of deficiency of a single micronutrient deficiency is relatively easy. However, milder forms of deficiency, particularly of multiple micronutrients concurrently, may be difficult to recognize clinically. Micronutrient status is likely to be affected by acute illness, owing to a combination of reduced intake and increased demand. Such increased demand may be due to catabolism resulting from the illness, subsequent anabolism and from disease-specific increased losses, for example loss from fistulae, diarrhoea and dialysis. As laboratory concentration of micronutrients may be perturbed in the acute phase, deficiencies in this group of patients can be particularly difficult to assess. It is increasingly being recognized that subclinical deficiencies of micronutrients may have detrimental effects, some of which are described in the following pages. As the assessment of micronutrients can be important in patients receiving supplementation, consideration is also given to aspects of micronutrient toxicity. Vitamin D is considered with bone metabolism in Chapter 31. Cobalt as a constituent of vitamin B12 is discussed, together with this vitamin, folate and iron, in Chapter 27. Iodine is essential in the formation of thyroid hormones (see Chapter 19). Vitamins are traditionally classified into those that are fat soluble (A, D, E and K) and the remainder, which are water soluble. This is probably still a useful distinction, as it predicts when deficiency is likely (e.g. fat-soluble vitamins in steatorrhoea), and is retained here. The fat-soluble vitamins A, E and K are structurally different, but are all non-polar, water-insoluble lipids. Absorption of each of them requires micelle formation in the gut lumen, for which bile salts are necessary. Dietary vitamin A occurs in a variety of forms which can be broadly divided into preformed vitamin A (retinol) and carotenoids, which are cleaved in the body to give vitamin A (see Fig. 10.3). In the UK, up to 75% of dietary vitamin A is preformed, derived from foods such as fortified cereals, margarine and dairy products, fish-liver oil and multivitamin preparations. Carotenoids are found in plants; the most important in the UK diet is β-carotene, which is found in carrots, dark green leafy vegetables, pumpkins and mangoes. Retinol is transported in the bloodstream bound to retinol-binding protein (RBP), which in turn forms a complex with thyroid-binding pre-albumin. Protein-energy malnutrition can result in reduced synthesis of RBP and thus impaired retinol transport from the liver, producing a functional vitamin A deficiency, even in the presence of adequate liver reserves. β-Carotene undergoes oxidative fission in the intestine to give retinal (see Fig. 10.3), and hence retinol. The cleavage of provitamin A carotenoids appears to be inhibited when vitamin A stores are high, hence, toxicity from ingestion of plant sources is rare. Ingestion of excess preformed vitamin A leads to its absorption and hepatic storage. The best understood of the actions of vitamin A is probably its role in vision. The 11-cis form of retinal is a component of the visual pigment rhodopsin, which is found in the rods of the retina. Light causes the retinal to change to the all-trans form (see Fig. 10.3) and this triggers a series of conformational and other changes, the end result being that an electrical signal is transmitted to the cortex of the brain and is perceived as light. A similar mechanism operates in the cones of the retina. Vitamin A is also necessary for growth and for normal development and differentiation of tissues. These actions are mediated by retinoic acid, which is present in foods in only small amounts but can be manufactured in the body from retinol. It modulates gene expression by activation of nuclear receptors, of which there are two groups: retinoic acid receptors (RAR) bind all-trans-retinoic acid (and some other retinoids); retinoid X receptors (RXR) bind 9-cis-retinoic acid. The latter interact with other receptors (e.g. those for vitamin D and thyroid hormones), and in the absence of retinoic acid can also act as repressors of gene expression. Other metabolic roles of vitamin A include carrying mannosyl units in the synthesis of hydrophobic glycoproteins and the retinoylation of proteins, for example the regulatory subunits of cAMP protein kinases. Vitamin A deficiency is common in some developing countries, particularly in children, and is the single most common preventable cause of blindness in the world. An early symptom is night blindness, because of inadequate amounts of 11-cis-retinal in the retina. However, deficiency also has important effects in mucus-secreting epithelia and so there are other consequences for the eyes. Keratinizing squamous metaplasia in the conjunctiva causes conjunctival xerosis and this may be followed by the appearance of white plaques of desquamated thickened epithelium, known as Bitot’s spots. Similar metaplasia in the cornea can lead to ulceration, which may progress to scarring and consequent blindness. Changes in other mucus-secreting epithelia, for example in the respiratory and gastrointestinal tracts, probably account in part for the lowered resistance to infections reported in vitamin A deficiency. In such areas of the world, public health programmes aimed at administering large doses of vitamin A have been successful in reducing morbidity and mortality associated with its deficiency, particularly in young children. Acute ingestion of large amounts of preformed vitamin A can result in raised intracranial pressure, with headaches, nausea, vomiting and visual disturbances. Chronic overdosage is associated with bone damage including increased bone resorption, spontaneous fracture and sometimes raised plasma calcium concentration and alkaline phosphatase activity. Liver damage, hair loss and skin changes have also been reported. Retinol is teratogenic (or, at least, the synthetic retinoids used in the treatment of certain dermatological conditions are) and so, in the UK, pregnant women are advised against self-medication with vitamin A and against consumption of liver and products made from it. A high intake of β-carotene results in an orange-yellow appearance of the plasma, body fat and skin, but is not usually regarded as harmful in the short term. However, there may be an increased incidence of malignancy in people taking β-carotene supplements, particularly among heavy smokers. There are eight very similar compounds that have vitamin E activity, four tocopherols and four tocotrienols. The most active is the natural isomer of α-tocopherol, which accounts for about 90% of the vitamin E present in human tissues. Its structure is shown in Figure 10.4. It appears to be the major lipid-soluble antioxidant in cell membranes, acting to prevent the peroxidation of unsaturated fatty acids by free oxygen radicals. α-Tocopherol, but not its related tocopherols and tocotrienols, also has actions that are independent of its antioxidant properties. It modulates the transcription of certain genes, inhibits platelet aggregation and vascular smooth muscle proliferation and has an effect on cell signalling in the immune system. The nutritional requirement for vitamin E is approximately proportional to the intake of polyunsaturated fatty acids. However, since foods rich in these (e.g. vegetable oils) also tend to contain large amounts of vitamin E, deficiency states are rare. Vitamin E is not easily transported across the placenta and the first vitamin E deficiency state to be firmly established was in premature infants, who developed haemolytic anaemia, thrombocytosis and oedema. In children and adults who are unable to absorb or utilize vitamin E adequately (e.g. in cystic fibrosis or abetalipoproteinaemia), a progressive spinocerebellar degeneration may develop. The full syndrome consists of ataxia of the limbs, loss of position and vibration senses, absent deep tendon reflexes and pigmentary degeneration of the retina. Treatment with vitamin E supplements can prevent these neurological features or, if they are already present, arrest or even reverse them. For α-tocopherol to be made available to the tissues from very low density lipoprotein, it must first bind to hepatic α-tocopherol transfer protein. Individuals with a deficiency of this transfer protein develop a very similar clinical syndrome (ataxia with vitamin E deficiency, AVED). There is some evidence that increased tissue concentrations of antioxidants, in particular vitamin E, may protect against conditions such as cancer and ischaemic heart disease, but this remains controversial. Vitamin E supplements have also been taken to improve general well-being and sexual performance, but the only firm conclusion that can be drawn is that quite high intakes of vitamin E are non-toxic, although very high intakes may antagonize vitamin K and potentiate anticoagulant therapy. The structures of the two forms of vitamin K and two vitamin K antagonists are shown in Figure 10.5. Vitamin K is involved in the post-translational modification of the blood coagulation factors II (prothrombin), VII, IX and X. A vitamin K-dependent enzyme system catalyses the carboxylation of the first ten amino-terminal glutamate residues in prothrombin to γ-carboxyglutamate (Gla). This carboxylation facilitates the binding of calcium, which is necessary for prothrombin to bind to platelet phospholipid, which is in turn necessary for the conversion of prothrombin to thrombin in the final common pathway of the clotting cascade. A similar process of γ-carboxylation occurs in factors VII, IX and X to form sites with a high affinity for calcium, and this is also known to occur in some clotting inhibitors (protein C and protein S). It also occurs in some proteins outside the clotting system, for example in osteocalcin and the matrix Gla protein in bone, in nephrocalcin in the renal cortex and in the product of the growth arrest specific gene (GAS6), which regulates differentiation and development in nervous tissue and apoptosis in other tissues. The main dietary form of vitamin K is phytomenadione (K1), with leafy green vegetables being the richest source, although other vegetable sources are important in most diets. Menaquinones (K2) produced by the intestinal bacteria may also be important, since while circulating vitamin K is mainly phytomenadione, hepatic reserves are mainly menaquinones. Vitamin K deficiency in its severest form leads to a bleeding syndrome, but dietary deficiency is rare after the first few months of life, unless there is an underlying disease affecting the absorption or utilization of the vitamin. However, vitamin K is undetectable in the blood of new-born babies, their hepatic reserves are low compared with adults and human breast milk contains barely adequate amounts. Thus, deficiency may present in infants as vitamin K deficiency bleeding (VKDB), within the first 24 h from the time of birth (early), between two and seven days (classic) or after seven days (late). Intracranial haemorrhage is rare in the early form, but occurs in more than 50% of babies with the late form and is likely to lead to death or severe neurological sequelae. The administration of vitamin K in the neonatal period abolishes the risk of deficiency. A single intramuscular injection of 1 mg of phytomenadione is highly effective for this purpose but suspicions, which now appear to be unfounded, were raised that its use led to an increased risk of childhood malignancy, particularly leukaemia. This adverse association has not been found for the administration of oral vitamin K, but the latter requires a multi-dose regimen. The current UK recommendation is that either intramuscular or oral vitamin K should be offered to all neonates after informed discussion with the parents. Toxicity from naturally occurring K vitamins would appear to be rare, even in quite high doses. However, the use of synthetic preparations of menadione for nutritional purposes is best avoided, as serious unwanted effects have been recorded (e.g. haemolysis and liver damage in neonates). The water-soluble vitamins are polar compounds that, with the exception of vitamin B12, tend to be absorbed rapidly from the upper small intestine, mainly through either sodium-dependent active transport or carrier-mediated diffusion. Vitamin B12 and folate are discussed in Chapter 26. The physiologically active form of thiamin (vitamin B1) is thiamin pyrophosphate (TPP). This functions as a cofactor in the conversion of pyruvate to acetyl-CoA by the pyruvate dehydrogenase complex and in the conversion of 2–oxoglutarate to succinyl-CoA by the 2–oxoglutarate dehydrogenase complex. It acts as a cofactor to the enzyme transketolase in the pentose phosphate pathway and plays a part in the metabolism of branched chain amino acids. Thiamin also has non-cofactor roles: thiamin triphosphate is found in nerve and muscle cells, where it activates membrane ion channels, possibly by phosphorylating them. Thiamin is found in most foodstuffs, but wheatgerm, oatmeal and yeast are particularly rich sources. However, the body store of about 30 mg is only 30 times the daily requirement and so an inadequate diet is likely to lead to deficiency of thiamin sooner than of any other vitamin. Thiamin deficiency used to be widespread in areas where rice formed a major part of the diet, resulting from the consumption of polished rice (from which the husk had been removed). In present times, thiamin deficiency is seen more typically in alcoholics (alcohol decreases the absorption of thiamin). Diets high in carbohydrate require more thiamin for their assimilation than diets high in fat, and subclinical deficiency may be unmasked by refeeding with a carbohydrate-rich diet. Patients on long-term renal replacement treatment may become deficient in thiamin (and other water-soluble vitamins) unless given supplements. Most of the clinical features of the disease beriberi respond to treatment with thiamin, suggesting that it is mainly due to thiamin deficiency. Two forms are described, one in which there is peripheral neuropathy, muscle weakness, general fatigue and reduced attention span (‘dry’ beriberi) and one in which there is also oedema and heart failure (‘wet’ beriberi). Some of these features may be the result of coexisting protein deficiency. Patients with thiamin deficiency (commonly alcoholics) may present with a neuropathy and cardiomyopathy, but may also develop an encephalopathy, the Wernicke–Korsakoff syndrome. Wernicke encephalopathy and Korsakoff psychosis were originally described as two separate conditions, but now appear to be the acute and chronic manifestations, respectively, of a single condition. Wernicke encephalopathy has an acute onset, with ophthalmoplegia, nystagmus, ataxia and stupor or apathy. It is a medical emergency: the ophthalmoplegia, ataxia and lowered consciousness respond, in most cases within two days, to treatment with thiamin but, in up to 80% of cases, the mental changes fail to resolve completely and Korsakoff psychosis develops. Once established, this responds only slowly or not at all to thiamin treatment. The main features are retrograde amnesia, difficulty in assimilating new ideas and confabulation. Susceptibility to the development of this syndrome in thiamin deficiency may be greater in people who have a genetic variant of transketolase that binds thiamin less avidly than usual. Thiamin is relatively non-toxic and can be given safely if deficiency is suspected. However, anaphylaxis has occurred after parenteral administration, and a wide variety of toxic effects has been described in adults with chronic intakes in excess of 50 mg/kg body weight or 3 g/24 h. Riboflavin (Vitamin B2
Clinical biochemistry of nutrition
INTRODUCTION
NUTRITIONAL REQUIREMENTS
The ‘correct’ intake
Energy
Carbohydrate
Fat
Protein
Micronutrients
Vitamins
Fat-soluble vitamins
Vitamin A
Vitamin E
Vitamin K
Water-soluble vitamins
Thiamin
Riboflavin
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree