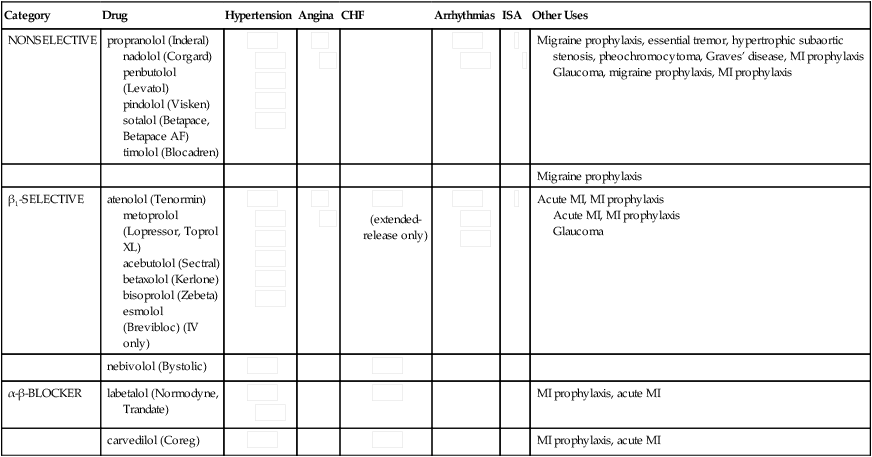
Chapter 20 All β-blockers have a similar mechanism of action—competitive blockade of the β-adrenergic receptor. This results in decreased heart rate, myocardial contractility, blood pressure, and myocardial oxygen demand. β-Blockers also reduce the metabolic (glycogenolytic, lipolytic), myocardial stimulant, vasodilator, and bronchodilator actions of catecholamines, and they suppress renin release (Table 20-2). TABLE 20-2 Comparison of Adrenergic Receptors Clinically significant differences between β-blockers have been noted. These agents are classified by their β-blocking selectivity (β1– or β2-α [β-blocking ability]), membrane-stabilizing activity (MSA), ISA, and pharmacokinetics. See Table 20-3 for characteristics of individual agents. TABLE 20-3 Characteristics of Individual β-Blockers ISA, Intrinsic sympathomimetic activity; MSA, membrane-stabilizing activity.
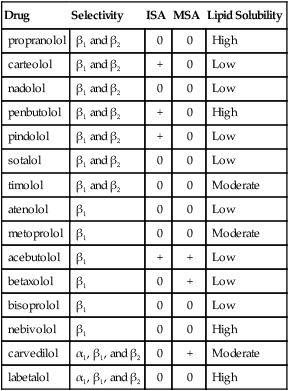
β-Blockers
Class
Subclass
Generic Name
Trade Name
β-Adrenergic blockers
Nonselective
propranolol ![]()
Inderal, InnoPran XL, generic
nadolol
Corgard, generic
penbutolol
Levatol
pindolol
Viskin, generic
sotalol
Betapace, Sotalol, generic
timolol
Betimol, Blocadren, generic
β 1-Selective
atenolol
Tenormin, generic
metoprolol
Lopressor, Toprol XL, generic
acebutolol
Sectral, generic
betaxolol
Kerlone, Betoptic, generic
bisoprolol
Zebeta, generic
nebivolol
Bystolic
esmolol
Brevibloc, generic
α-β-Blocker
labetalol
Trandate, generic
carvedilol
Coreg, generic
Mechanism of Action
Receptor
Site
Effect of Stimulation
α1
Smooth muscle in blood vessels
Vasoconstriction
Stomach, intestine
Decreased motility and tone
Kidney
Increased renin secretion
Liver
Gluconeogenesis
α2
Smooth muscle in blood vessels
Vasodilation
β1
Cardiac
Increased rate and force of contraction
Kidney
Increased renin secretion
β2
Bronchial, vascular, coronary arteriole, uterine smooth muscle, skeletal muscle
Vasodilation
Pancreas
Decreased secretion
Liver
Gluconeogenesis
Drug
Selectivity
ISA
MSA
Lipid Solubility
propranolol
β1 and β2
0
0
High
carteolol
β1 and β2
+
0
Low
nadolol
β1 and β2
0
0
Low
penbutolol
β1 and β2
+
0
High
pindolol
β1 and β2
+
0
Low
sotalol
β1 and β2
0
0
Low
timolol
β1 and β2
0
0
Moderate
atenolol
β1
0
0
Low
metoprolol
β1
0
0
Moderate
acebutolol
β1
+
+
Low
betaxolol
β1
0
+
Low
bisoprolol
β1
0
0
Low
nebivolol
β1
0
0
High
carvedilol
α1, β1, and β2
0
+
Moderate
labetalol
α1, β1, and β2
0
0
High
β-Blockers
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


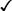
 (extended-release only)
(extended-release only)