and Natalia Buza1
(1)
Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
Keywords
Cervical neoplasmsTumorlike lesionsSquamous cell carcinomaAdenocarcinomaMullerian adenosarcomaMalignant mixed Mullerian tumorLymphomaIntroduction
Squamous cell carcinoma is the most common cervical malignancy and is caused by high-risk human papillomavirus (HPV) with viral DNA integration into the host cell genome [1]. Most tumors are conventional squamous cell carcinomas of either keratinizing or nonkeratinizing large cell type. Common histological variants include verrucous, basaloid, papillary, lymphoepitheliomatous, and spindled squamous carcinomas. Invasive adenocarcinoma of the cervix now represents 10–20 % of all cervical cancers. Similar to squamous carcinoma, high-risk HPV, particularly type 18, is the causal agent for most of the cases. The histological spectrum of cervical adenocarcinomas includes usual endocervical adenocarcinoma, endometrioid adenocarcinoma, gastrointestinal-type mucinous carcinoma and its variants including minimal deviation adenocarcinoma (adenoma malignum), villoglandular adenocarcinoma, adenoid basal carcinoma, adenosquamous carcinoma, serous carcinoma, and clear cell carcinoma. Malignant stromal tumors of the cervix are uncommon. Many reactive, metaplastic, and tumorlike conditions may simulate various malignant processes of the cervix.
Common scenarios for intraoperative consultation include evaluation for the presence of malignancy of an unexpected cervical mass and/or assessment of the extent of tumor involvement—depth of tumor invasion and margin status—and lymph node metastasis. Intraoperative pathologic evaluation of pelvic lymph nodes may be requested before hysterectomy to rule out advanced disease. More recently frozen section evaluation of sentinel lymph nodes has also been introduced to the surgical management of cervical malignancy. In young patients, the most common reason for performing frozen section evaluation of a cervical cone excision, trachelectomy, or biopsy is to maximize the chance of preserving fertility by doing limited surgery for an early cervical cancer.
Tumorlike Conditions of the Cervix
Endocervical Polyp
Usually single and less than 1 cm in size (Fig. 3.1).
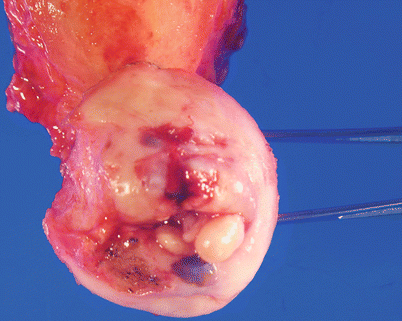
Fig. 3.1
Endocervical polyp. White-tan polypoid lesions of several millimeters with adjacent mucosal erosion and hemorrhage in this example
Histologically, the polyp consists of varying proportions of squamous and endocervical glandular epithelium and stromal components.
The overall appearance can be adenomatous, cystic, fibrous, or inflammatory (Fig. 3.2).
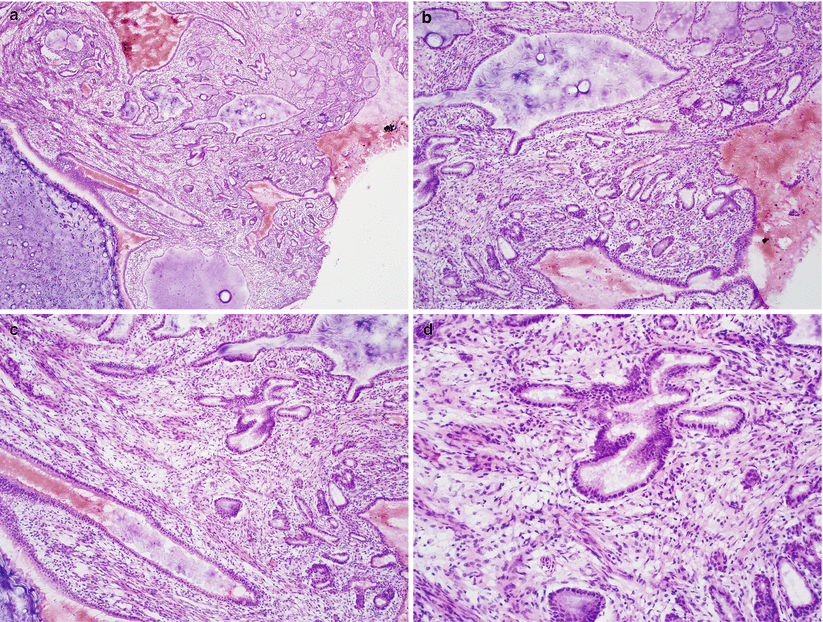
Fig. 3.2
Endocervical polyp. Note the polypoid configuration, edematous stroma (a, b) and variable number of benign endocervical glands (c, d)
Differential diagnosis
Mullerian adenosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
Unlike adenosarcoma, benign endocervical polyps are characterized by absence of periglandular stromal cell condensation and papillary intraglandular protrusions and lack of epithelial atypia and stromal mitotic activity.
Tunnel Clusters
Localized proliferation of endocervical glands, commonly seen in multigravida women.
Type A tunnel cluster is noncystic, consisting of crowded endocervical glands with round borders. Irregular glands with mild atypia may be seen, but mitotic activity is generally absent.
Type B tunnel cluster consists of round clusters of packed glands with cystic dilatation that are covered by flattened epithelial cells.
Both types may involve deeper cervical stroma.
Differential diagnosis
Minimal deviation adenocarcinoma (MDA)
Diagnostic pitfalls/key intraoperative consultation issues
Tunnel clusters can be diagnostically separated from MDA primarily by absence of significant cytological atypia, absence of desmoplastic stromal response, and their overall lobular growth pattern and sharp lesional border.
Deep Nabothian Cyst
Deeply seated, cystically dilated, mucin-filled endocervical glands (Fig. 3.3).
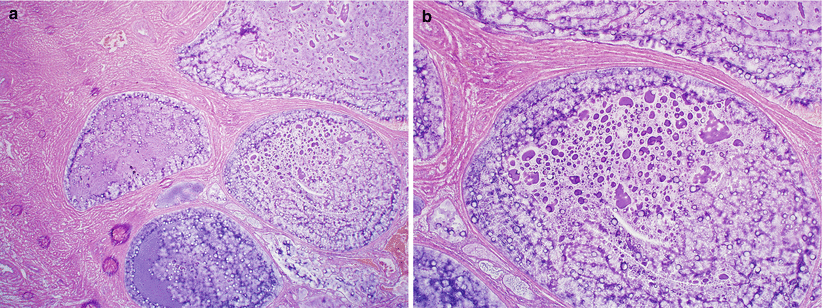
Fig. 3.3
Deep nabothian cyst. Deeply seated, cystically dilated endocervical glands filled with mucin (a, b)
Extravasated mucin pools may be present.
Cysts are lined by bland columnar or flattened endocervical glandular epithelium.
Differential diagnosis
Minimal deviation adenocarcinoma (MDA)
Diagnostic pitfalls/key intraoperative consultation issues
Lack of a mass lesion, and absence of infiltrative glands and significant cytological atypia separates nabothian cysts from minimal deviation adenocarcinoma.
Lobular and Diffuse Laminar Endocervical Glandular Hyperplasia [2, 3]
Benign mucinous glandular proliferations, in a lobular or diffuse laminar pattern.
May be deeply seated lobular mass lesions involving cervical stroma, exceeding 1 cm in size (Fig. 3.4).
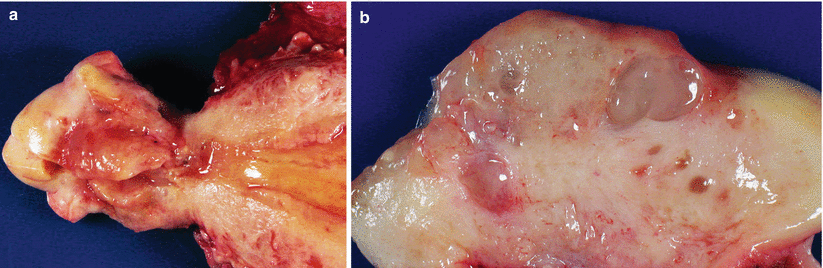
Fig. 3.4
Diffuse laminar endocervical glandular hyperplasia. Note the ill-defined, mucoid mass lesion involving the endocervix (a, b)
Both types show crowded hyperplastic glands lined by mucin-producing endocervical glandular epithelial cells.
Both may have mild cytological atypia and low mitotic activity (2 per 10 high-power fields.
In diffuse laminar hyperplasia, the lesion shows a band-like distribution below the endocervical mucosal surface. Although confluent in growth pattern, the demarcation from the stroma is sharp and smooth (Fig. 3.5).
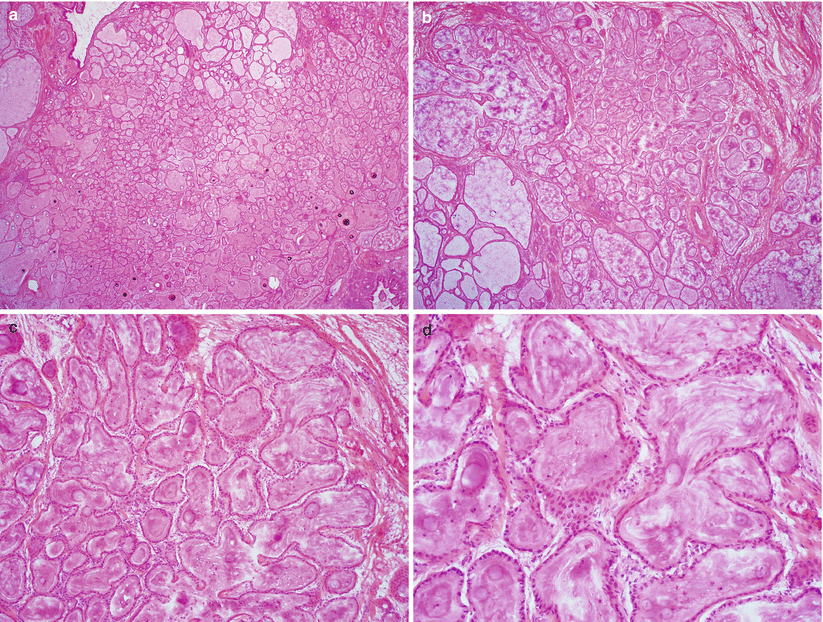
Fig. 3.5
Diffuse laminar endocervical glandular hyperplasia. Note diffuse hyperplastic proliferation of endocervical glands (a) well-demarcated from the adjacent endocervical stroma (b, c) and absence of cytological atypia or mitotic activity (d)
Differential diagnosis
Mucinous endocervical adenocarcinomas, including minimal deviation adenocarcinoma, intestinal-type mucinous adenocarcinoma, and mucinous adenocarcinoma NOS
Diagnostic pitfalls/key intraoperative consultation issues
Endocervical glandular hyperplasia may be separated from endocervical adenocarcinomas by its superficial location, non-infiltrative border, and absence of significant cytological atypia and desmoplastic stromal response.
Microglandular Hyperplasia (MGH) [4–6]
Incidental microscopic finding or may be a grossly polypoid lesion.
Polypoid, reticular, cribriform, and rarely solid proliferation of closely packed small- to medium-sized endocervical glands with conspicuously neutrophil-rich stroma (Fig. 3.6).
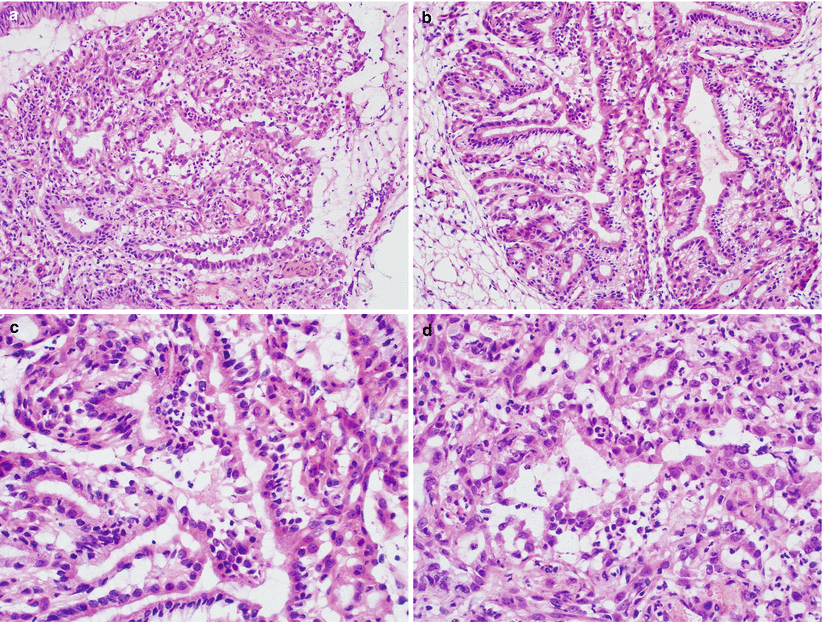
Fig. 3.6
Microglandular hyperplasia. Note the closely packed small- to medium-sized endocervical glands (a, b), uniform endocervical cells with noticeable reserve cell layer in some glands (c) and the presence of neutrophils in the stroma (d)
Relatively uniform endocervical glandular cells with subnuclear vacuoles, associated squamous metaplasia, often with reserve cell hyperplasia imparting on a double-layered epithelial appearance.
Mild nuclear atypia may be present.
Mitoses are usually rare but occasional cases may show higher mitotic activity (rarely up to 11 mitoses/10 HPF).
Differential diagnosis
Clear cell carcinoma
Endometrioid adenocarcinoma—endometrial or endocervical primary
Mucinous adenocarcinoma—endometrial or endocervical primary
Diagnostic pitfalls/key intraoperative consultation issues
Absence of infiltrative growth and lack of significant cytological atypia separate florid microglandular hyperplasia from adenocarcinoma.
Endometrial microglandular adenocarcinoma, unlike MGH, is characterized by absence of a reserve cell layer, often presence of significant cytological atypia and continuity with endometrial rather than endocervical tissue
Chronic Endocervicitis
Intense lymphoid hyperplasia with marked squamous metaplasia and secondary lymphoid follicle formation.
Surface papillary epithelial proliferation with associated lymphoid follicles (papillary cervicitis) may be seen.
Rarely, a lymphoid germinal center may be confused with high-grade squamous intraepithelial lesion involving endocervical glands, due to its pleomorphic lymphoid cell population and frequent mitotic activity.
Lymphoma-Like Lesions [7, 8]
Adult patients.
May present with vaginal bleeding or discharge.
Thickened or erythematous, friable mucosa.
Band-like subepithelial mixed lymphoid infiltrate consisting of reactive large centroblasts or immunoblasts and mature plasma cells.
Lack of a mass lesion and deep involvement separates it from true lymphoma.
Arias-Stella Reaction [9]
Generally focal, may appear papillary on gross examination.
Enlarged glandular cells with abundant clear cytoplasm and apically located hyperchromatic nuclei (hobnail cells).
The lesion may closely resemble clear cell carcinoma. The overall normal glandular distribution, degenerative nature of nuclei, absence of mitoses, and concurrent pregnancy or hormone use are typical features of Arias-Stella reaction.
Mesonephric Hyperplasia [10–12]
Typically located at the lateral aspect of cervix.
Glandular proliferation in a lobular or irregular distribution around a centrally located duct.
Glands are lined by cuboidal or low columnar epithelial cells.
No significant nuclear atypia or mitotic activity.
Characteristic intraluminal dense eosinophilic secretion is present.
Pseudostratification, papillary tufting, and bridging are compatible with benign mesonephric hyperplasia.
Differential diagnosis
Mesonephric adenocarcinoma
Diagnostic pitfalls/key intraoperative consultation issues
Diffuse mesonephric hyperplasia may pose a significant diagnostic challenge on frozen section.
Significant cytological atypia, desmoplastic stromal response, presence of luminal necrotic debris, perineural invasion, lymphovascular invasion, and haphazard infiltrative growth are features of mesonephric carcinoma.
Endocervicosis [13]
Patients may have a history of uterine surgery (e.g., caesarean section).
Nodular to cystic mass lesion consisting of variably sized and shaped glands infiltrating the outer cervical stroma.
Columnar to flattened endocervical glandular cells without significant cytological atypia and absence of mitotic activity.
Stromal response to extravasated mucin may be present.
Differential diagnosis
Minimal deviation adenocarcinoma
Metastatic well-differentiated adenocarcinoma, particularly when the lesion also involves adjacent organs including bladder, cul-de-sac, and vaginal apex
Diagnostic pitfalls/key intraoperative consultation issues
Separation of endocervicosis from an adenocarcinoma is based on lack of cytological atypia, mitosis, and stromal response.
Endometriosis
Frequent lesion of the cervix.
Deep cervical endometriosis is usually associated with pelvic endometriosis.
Typical endometrial stroma with hemorrhage or hemosiderin pigment around proliferative-type endometrial glands.
Differential diagnosis
Cervical adenocarcinoma in situ
Diagnostic pitfalls/key intraoperative consultation issues
In contrast to cervical adenocarcinoma in situ, endometriosis lacks significant cytological atypia, nuclear stratification, apically located mitotic figures, and numerous apoptotic bodies.
Tubo-endometrial Metaplasia [14]
Common lesion of the cervix and may be related to a prior cervical procedure (e.g., loop excision).
Glands with tubal and/or endometrioid differentiation.
Oxyphilic changes with increased eosinophilic cytoplasm and enlarged nuclei may be present.
Absence of significant cytological atypia and mitotic activity and presence of tubal-type ciliated cells are helpful features separating tubo-endometrioid metaplasia from cervical adenocarcinoma in situ
Squamous Metaplasia
Very common condition involving the cervical transformation zone or an endocervical polyp.
Squamous metaplasia may be extensive, frequently involving endocervical glands, simulating squamous intraepithelial lesion or even invasive squamous cell carcinoma (Fig. 3.7).
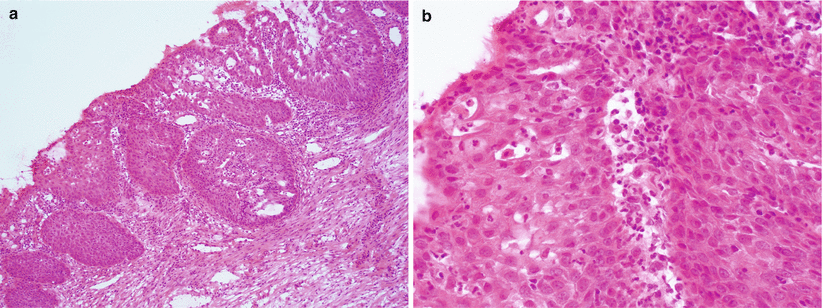
Fig. 3.7
Squamous metaplasia at the cervical transformation zone. Metaplastic squamous epithelium frequently extends into the endocervical glands (a). Note the absence of significant cytological atypia and lack of true stromal invasion (b)
Papillary immature squamous metaplasia may simulate papillary squamous cell carcinoma.
Frequently associated with marked inflammation.
Absence of cytological atypia and stromal invasion confirm the diagnosis.
Transitional Metaplasia
Typically occurs in peri- and postmenopausal women
Transitional-type epithelial cells with small, oval nuclei replacing the full thickness of squamous epithelium
Streaming of nuclei with frequent nuclear grooves
Absence of significant cytological atypia and mitotic activity
Differential diagnosis
High-grade squamous intraepithelial lesion (cervical intraepithelial neoplasia 3; CIN3)
Diagnostic pitfalls/key intraoperative consultation issues
Unlike transitional metaplasia, high-grade dysplasia is characterized by loss of epithelial polarity, marked cytological atypia with increased nuclear to cytoplasmic ratio, and mitotic activity, often with atypical mitoses.
Squamous Cell Carcinoma
Squamous Intraepithelial Lesion
The most common precursor lesion of cervical squamous carcinoma.
Almost always associated with human papillomavirus (HPV) infection, particularly high-risk HPV in high-grade intraepithelial lesion (HSIL).
HSIL mostly occurs in patients in their mid twenties to mid thirties.
Dysplastic squamous cells occupy at least 2/3 of the epithelium with loss of polarity in HSIL. Low grade intraepithelial lesion (LSIL) has dysplastic cells limited to the lower third of the epithelium.
Histological features include nuclear enlargement, high nuclear/cytoplasmic ratio, coarse chromatin, thickened nuclear membrane, and easily identifiable mitoses and atypical mitoses in HSIL (Fig. 3.8).
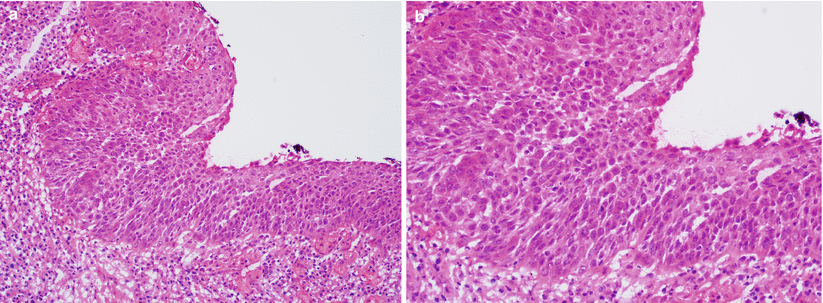
Fig. 3.8
High-grade squamous intraepithelial lesion (HSIL). Dysplastic squamous cells occupy approximately two-thirds of the epithelium (a, b)
Differential diagnosis
Immature squamous metaplasia
Papillary squamous metaplasia
Transitional metaplasia
Reactive epithelial changes
Invasive Squamous Cell Carcinoma (SCC)
Clinical features
Most common cervical cancer.
Associated with high-risk HPV infection and preceded by high-grade squamous intraepithelial lesion (HSIL).
Mean patient age is 55 years, but a third of cases occur in women less than 35 years of age.
Most common clinical symptoms are vaginal bleeding, discharge, and/or pelvic pain.
Gross pathology (Fig. 3.9)
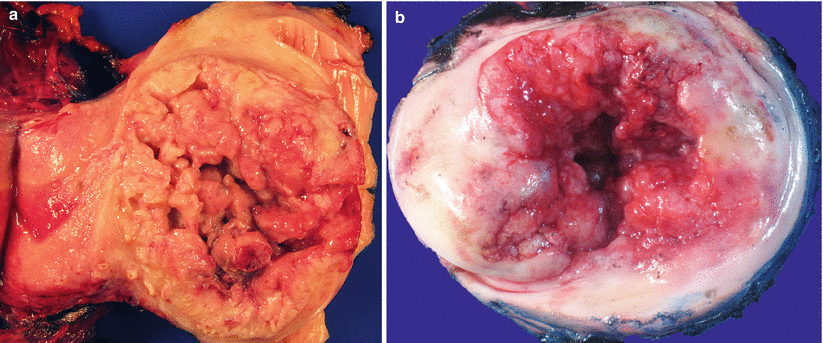
Fig. 3.9
Cervical invasive squamous cell carcinoma. Note the friable, exophytic mass lesions with hemorrhage and necrosis (a, b)
Pink, friable, commonly exophytic polypoid or papillary growth, or ulcerative surface lesion.
Almost always located at the cervical transformation zone.
Advanced tumors may show gross infiltration to various adjacent organs, e.g., parametrium, vagina, or uterine corpus.
Microscopic features [15] (Fig. 3.10)
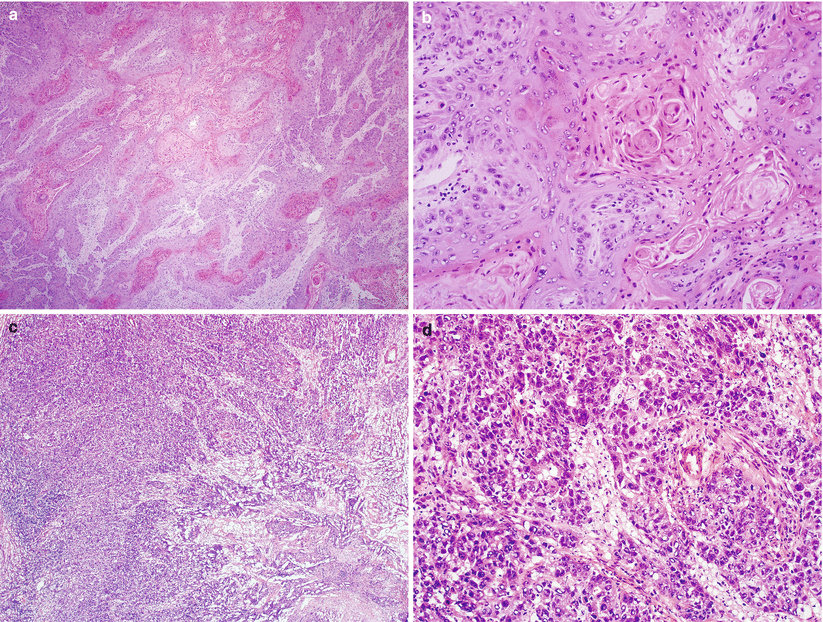
Fig. 3.10
Cervical invasive squamous cell carcinoma. Keratinizing well-differentiated (a, b) and poorly differentiated histological subtypes (c, d)
Proliferation of tumor cells in sheets and nests with anastomosing bands or single infiltrating cells.
Desmoplastic stromal response is common.
Tumor cells with variable amount of cytoplasm, large nuclei, and conspicuous nucleoli.
Keratinizing-type SCC
Shows obvious squamous maturation including keratin pearl formation
Nonkeratinizing-type SCC
Composed of medium-sized, atypical squamous cells without obvious keratin pearl formation, but individual tumor cell keratinization is often present
Superficially invasive SCC
Associated with HSIL and histologically similar to vulvar superficially invasive SCC (see Chap. 2)
Small irregular nests of highly atypical dysplastic epithelial cells, frequently with abrupt keratinization (paradoxical maturation)
Presence of stromal response, including stromal edema, desmoplasia, or prominent inflammatory changes
Variants of squamous carcinoma [16–23]
Verrucous carcinoma
Highly differentiated variant with undulating surface simulating condyloma or squamous papilloma.
Hyperkeratosis is common.
Minimal cytological atypia and low mitotic activity.
Multiple tumor blocks may be required for frozen section evaluation to reveal its pushing and bulbous invasive border.
Warty SCC
Well-differentiated variant with a warty surface and architecture similar to condyloma
Basaloid SCC
Aggressive tumor with characteristic peripheral palisading of primitive high-grade tumor cells.
Brisk mitotic activity and geographic necrosis.
Absence of stromal response.
Individual keratinization may be present, but keratin pearls are typically absent.
Papillary squamous/squamotransitional carcinoma
Resembles urothelial carcinoma with high-grade cells arranged in papillary fronds with stromal cores.
Adequate sampling for frozen section is important to include the tumor base to rule out possible coexistence of an invasive component.
Lymphoepitheliomatous variant
Syncytial growth of large tumor cells with vesicular nuclei, prominent nucleoli and surrounding marked inflammatory infiltrate
Spindle cell or sarcomatoid SCC
Rare variant of SCC with more aggressive clinical behavior.
Histologically sarcomatoid spindle cell proliferation that may merge with conventional SCC.
It should be separated from malignant mixed Mullerian tumor (MMMT).
In contrast to spindle cell SCC, MMMT has distinct squamous carcinomatous and sarcomatous components without merging between the two.
Differential diagnosis
HSIL extensively involving endocervical glands
Gestational trophoblastic lesions: epithelioid trophoblastic tumor, placental site nodule, and ectopic decidua
Clear cell carcinoma, solid variant vs. SCC with large amount of cytoplasmic glycogen
Neuroendocrine carcinoma, particularly small cell carcinoma vs. poorly differentiated SCC
Sarcomas vs. spindle cell/sarcomatoid SCC
Melanoma
Reactive and tumorlike conditions including exuberant squamous metaplasia, extensive inflammation, and pseudoinvasion of squamous epithelium induced by prior surgical procedure
Diagnostic pitfalls/key intraoperative consultation issues
Trachelectomy procedure may be used for management of early cervical cancer in young patients [24].
At least 5 mm proximal surgical margin clearance is recommended and the margin status should be reported on frozen section evaluation [25].
Frozen section evaluation of a cervical cone excision is labor intensive and time consuming and may compromise precise characterization of the tumor. If possible, discussion with submitting physician to defer for permanent sections should be attempted [26].
Presence of HSIL with focal transition to spindled invasive component is a helpful hint for the diagnosis of spindle cell SCC.
Absence of sharply demarcated carcinomatous and sarcomatous components separates spindle cell SCC from malignant mixed Mullerian tumor.
Microinvasive/superficially invasive SCC vs. HSIL involving endocervical glands.
Loss of peripheral palisading with markedly atypical cells, stromal response, and abrupt tumor cell keratinization (abnormal or paradoxical maturation) are features of invasion.
When in doubt, a frozen diagnosis of “high-grade squamous intraepithelial lesion, cannot rule out invasion” may be communicated to the surgeon.
Sentinel lymph node evaluation for cervical cancer [27, 28]
Sentinel lymph node evaluation may be performed at the time of hysterectomy for cervical cancer.
In the absence of uniform guidelines, grossly identifiable lymph nodes should be serially sectioned and submitted for frozen section evaluation followed by careful microscopic examination, particularly at the subcapsular sinus region to identify microscopic metastases (Fig. 3.11).
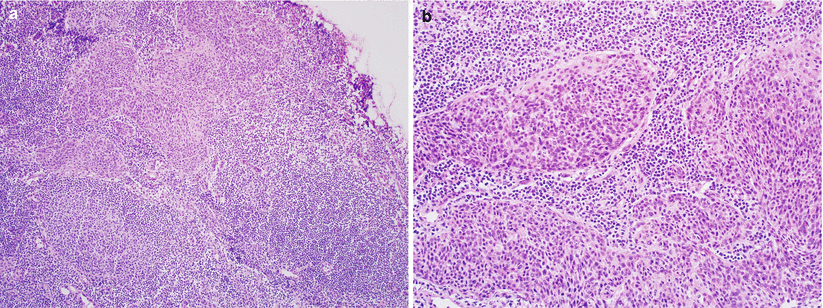
Fig. 3.11
Cervical squamous cell carcinoma metastasis in a regional lymph node (a, b)
Most common differential diagnosis of metastatic cervical carcinoma in a lymph node includes endosalpingiosis and endometriosis.
Both lesions lack malignant cytological atypia.
In contrast to subscapular sinus location of metastatic carcinoma, endosalpingiosis is found within the fibrous capsule of the lymph node [see Chap. 8] or nodal medullary sinuses.
Endometriosis may be found inside subscapular sinuses, and it is also associated with endometrial stroma around the glandular epithelium.
Other rare mimics include transported mesothelial cells within the nodal capsule and ectopic decidua in pregnant patients and should not be over-interpreted as metastatic disease.
Adenocarcinoma of the Cervix
Endocervical Adenocarcinoma In Situ (AIS) [24]
Mostly seen in patients in their thirties and forties.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


