7 • The central nervous system (CNS) comprising the brain and spinal cord • The peripheral nervous system (PNS) comprising the nerves which run between the CNS and other tissues, together with nerve ‘relay stations’ termed ganglia This chapter encompasses nerve cells and their supporting cells, focused on the histological structure of the peripheral nervous system and simple types of sensory receptors. Details of the supporting cells and arrangement of nervous tissue in the central nervous system are the subject of Ch. 20, while the structure of the highly specialised organs of sensory reception, eyes and ears, is presented in Ch. 21. FIG. 7.1 The neurone FIG. 7.2 Basic neurone types FIG. 7.3 Ultrastructure of the neurone (illustration (b) opposite) FIG. 7.4 Neurones and methods of study with light microscopy (illustrations (a–f) opposite) In the peripheral nervous system, all axons are enveloped by highly specialised cells called Schwann cells, which provide structural and metabolic support. In general, small-diameter axons (e.g. those of the autonomic nervous system and small pain fibres) are simply enveloped by the cytoplasm of Schwann cells; these nerve fibres are said to be non-myelinated. Large-diameter fibres are wrapped by a variable number of concentric layers of the Schwann cell plasma membrane forming a myelin sheath; such nerve fibres are said to be myelinated. Within the central nervous system, myelination is similar to that in the peripheral nervous system except that the myelin sheaths are formed by cells called oligodendrocytes (see Fig. 20.3). There are distinct chemical differences between central and peripheral myelin. In all nerve fibres, the rate of conduction of action potentials is proportional to the diameter of the axon; however, myelination greatly increases axon conduction velocity compared with that of a non-myelinated fibre of the same diameter. FIG. 7.5 Non-myelinated nerve fibres
Nervous tissues
Introduction
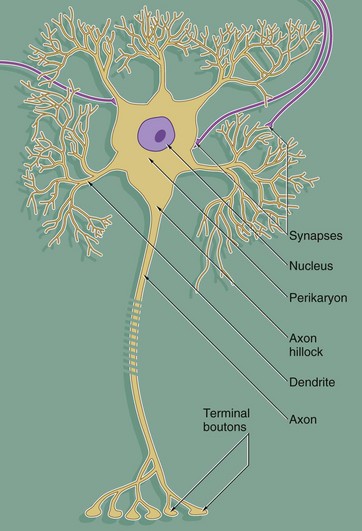
Despite great variation in size and shape in different parts of the nervous system, all neurones have the same basic structure as shown in this idealised diagram. The neurone consists of a large cell body containing the nucleus surrounded by cytoplasm known as the perikaryon. Processes of two types extend from the cell body, namely a single axon and one or more dendrites. Dendrites are highly branched tapering processes which either end in specialised sensory receptors (as in primary sensory neurones) or form synapses with neighbouring neurones from which they receive stimuli. In general, dendrites function as the major sites of information input into the neurone.
Each neurone has a single axon arising from a cone-shaped portion of the cell body called the axon hillock. The axon is a cylindrical process up to 1 metre in length, terminating on other neurones or effector organs by way of a variable number of small branches which end in small swellings called terminal boutons. Action potentials arise in the cell body as a result of integration of afferent (incoming) stimuli; action potentials are then conducted along the axon to influence other neurones or effector organs by release of neurotransmitter chemicals. Axons are commonly referred to as nerve fibres.
In general, the cell bodies of all neurones are located in the central nervous system; exceptions are the cell bodies of most primary sensory neurones and the terminal effector neurones of the autonomic nervous system where, in both cases, the cell bodies lie in aggregations called ganglia in peripheral sites.
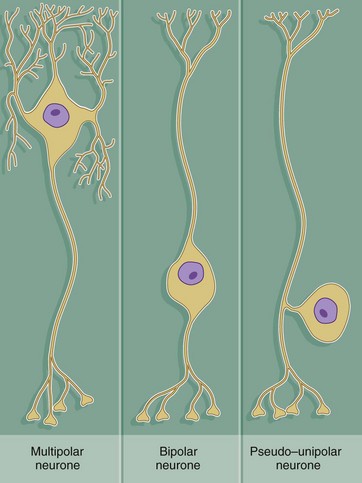
Throughout the nervous system, neurones have a wide variety of shapes which fall into three main patterns according to the arrangement of the axon and dendrites with respect to the cell body.
The most common form is the multipolar neurone in which numerous dendrites project from the cell body; the dendrites may all arise from one pole of the cell body or may extend from all areas of the cell body surface. In general, intermediate, integratory and motor neurones conform to this pattern.
Bipolar neurones have only a single dendrite which arises from the pole of the cell body opposite to the origin of the axon. These unusual neurones act as receptor neurones for the senses of smell, sight and balance. Most other primary sensory neurones are described as pseudo-unipolar neurones, since a single dendrite and the axon arise from a common stem of the cell body; this stem is formed by the fusion of the first part of the dendrite and axon of a bipolar type of neurone during embryological development.
As a general rule, neurone impulses are conveyed along dendrites towards the nerve cell body (afferent) while axons usually convey impulses away from the nerve cell body (efferent).
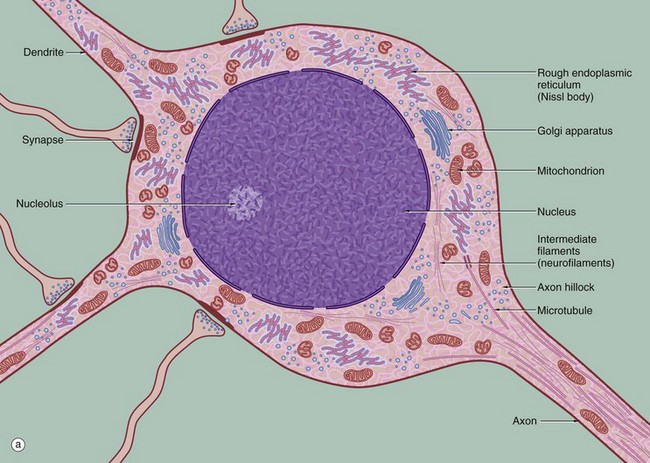
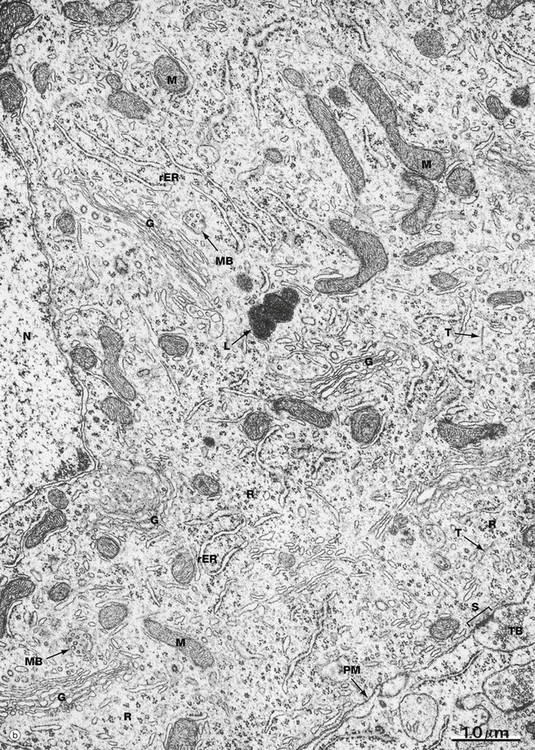
(a) Schematic diagram (b) EM ×19 000
The diagram (a) illustrates the main ultrastructural features of the neurone, in this case a multipolar neurone with an axon and two dendrites. The nucleus is large, round or ovoid and usually centrally located within the perikaryon. Reflecting the intense metabolic activity of the neurone (and consequent need to replace proteins which are rapidly turned over), the chromatin is completely dispersed and the nucleolus is a conspicuous feature.
The cytoplasm of the cell body contains large aggregations of rough endoplasmic reticulum which correspond to the Nissl substance of light microscopy (see Fig. 7.4); the rough endoplasmic reticulum extends into the dendrites but not into the axon hillock or axon. Rough endoplasmic reticulum is a much more prominent feature in large neurones, such as somatic motor neurones, than in smaller neurones such as those of the autonomic nervous system. A diffuse Golgi apparatus is found adjacent to the nucleus. Smooth endoplasmic reticulum is not a prominent feature of the perikaryon, but tubules, cisternae and vesicles are prominent in the axon and dendrites. The mitochondria of the perikaryon are numerous and have the usual rod-like appearances; those of the axon are extremely slender and elongated.
Neurones are very metabolically active and expend much energy in maintaining ionic gradients across the plasma membrane. Neurones synthesise neurotransmitter substances or their precursors in the perikaryon from where they are transported along the axon to the synapse to be released when appropriately stimulated.
Numerous intermediate filaments (neurofilaments) and microtubules are arranged in parallel bundles throughout the perikaryon and along the length of the axon and dendrites. The electron micrograph (b) shows part of the cell body of a neurone and includes a portion of the nucleus N. At the lower right, part of the neuronal plasma membrane PM, is seen, including a synapse S with the terminal bouton TB of an adjacent neurone. Features of the cytoplasm of the perikaryon are areas of rough endoplasmic reticulum rER, free ribosomes R and scattered mitochondria M. An extensive Golgi apparatus G is represented by several stacks of flattened membranous cisternae. Associated with the Golgi are several multivesicular bodies MB, which are involved in transport to other organelles including lysosomes L. Microtubules T can be identified in oblique section, but neurofilaments are not readily identifiable.
(a, b) H&E (HP) (c) Luxol fast blue, H&E (HP) (d) Gold method (HP) (e) Gold/toluidine blue (HP) (f) Immunohistochemistry for neurofilament protein (HP) (g) Spread preparation, gold method (MP) (h) Golgi-Cox (HP)
Special staining techniques are required to show the rich structural detail of the nervous system. Heavy metal impregnation techniques with gold and silver are valuable in the study of neurone morphology and were widely employed by the pioneers of neuroanatomy such as Cajal and Golgi, from whom they take their names. Likewise, spread preparations often permit the examination of complete neurones and their cytoplasmic processes. Immunohistochemistry can also be used to identify neurone-specific proteins (e.g. neurofilament protein).
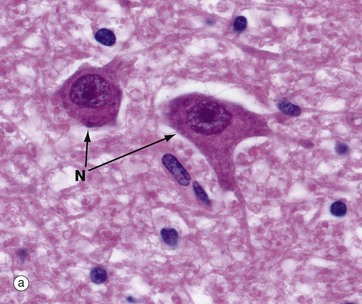
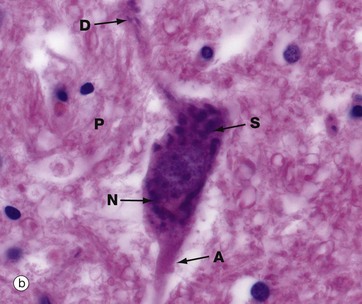
Micrographs (a) and (b), stained with H&E, show neurones N in the central nervous system; the nuclei are huge in comparison with those of surrounding support cells. Dispersed chromatin and prominent nucleoli reflect a high level of protein synthesis. Neurones have extensive cytoplasm which is basophilic (blue stained) due to extensive ribosomal RNA. Many of the ribosomes are associated with rough endoplasmic reticulum, found gathered in patches called Nissl substance S, which extends into dendrites D but not the axon A. In H&E preparations, virtually no detail can be seen of cytoplasmic processes, which merge into a fibrillary background termed the neuropil P composed of both nerve cell and support cell processes.
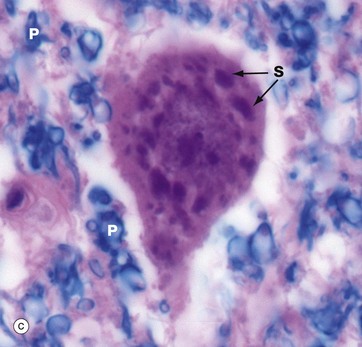
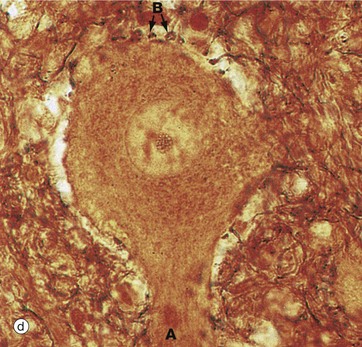
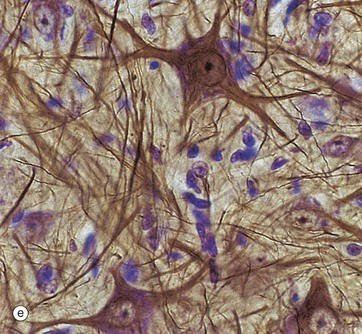
In micrograph (c), the Nissl substance is seen as dark purple material giving the neuronal cytoplasm a mottled appearance. Here the myelin (see below) is stained blue, demonstrating the structure of the neuropil P. A very similar neurone is shown in micrograph (d) using a heavy metal impregnation technique that highlights small axons. Numerous axons from other neurones with tiny terminal boutons B can be seen forming synapses with the cell body.
Micrograph (e) employs another gold method providing excellent detail of neuronal shape and showing the cytoskeleton in the dendrites and axons; the blue counter-stain demonstrates the nuclei of surrounding support cells. Note that detail in the neuronal processes is lost as they pass out of the plane of focus.
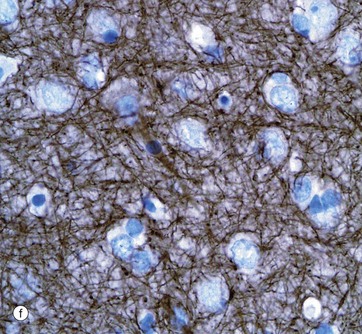
Micrograph (f) shows an area of the brain stained with an antibody to neurofilament protein. This highlights the complex network of axons within the neuropil between neurones. Neurones are seen as clear spaces containing a nucleus in this preparation.
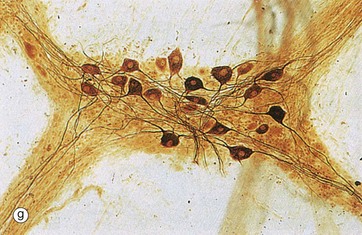
Spread preparations, as shown in micrograph (g), also outline the complexity of nerve cell processes, both axons and dendrites. Here, neurones in a small peripheral ganglion and their main cytoplasmic processes are clearly delineated.
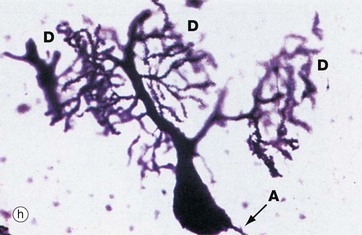
Micrograph (h) illustrates a very thick section stained by a silver impregnation method and shows a Purkinje cell in the cerebellar cortex. These cells have a single small axon A at one pole and an extraordinary finely branching dendritic tree D at the other pole. Note that the base of the dendritic system is in this case much larger than that of the axon.
Myelinated and Non-Myelinated Nerve Fibres
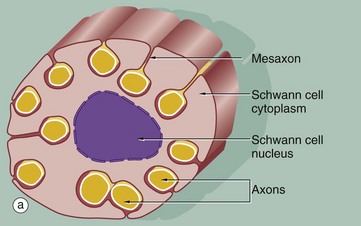
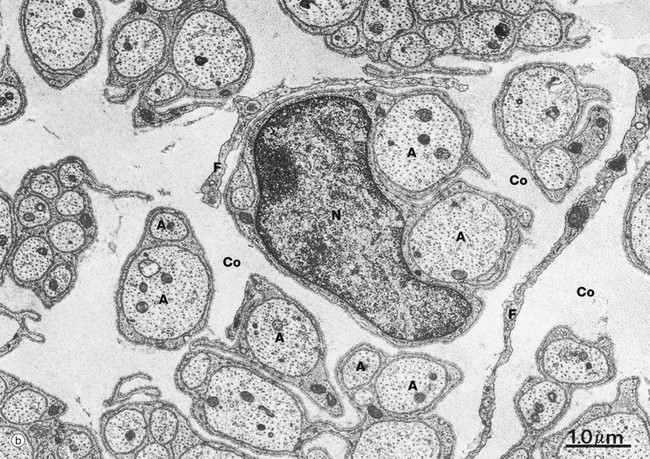
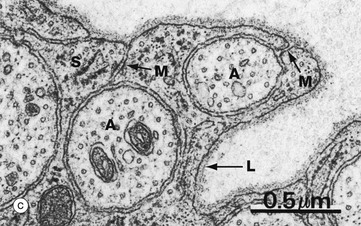
(a) Diagram (b) EM ×15 000 (c) EM ×36 000
The relationship of non-myelinated axons with their supporting Schwann cell is illustrated in diagram (a). One or more axons become longitudinally invaginated into the Schwann cell so that each axon is embedded in a channel, invested by the Schwann cell plasma membrane and cytoplasm. The Schwann cell plasma membrane becomes apposed to itself along the opening of the channel, thus effectively sealing the axon within an extracellular compartment bounded by the Schwann cell. The zone of apposition of the Schwann cell membrane is called the mesaxon. Note that more than one axon may occupy a single channel within the Schwann cell. Each Schwann cell extends for only a short distance along the nerve tract, and at its termination the ensheathment is continued by another Schwann cell with which it interdigitates closely end to end.
At low magnification in micrograph (b), non-myelinated axons A of various sizes are seen ensheathed by Schwann cells S; one of the Schwann cells has been sectioned transversely through its nucleus N. Note the variable number of axons enclosed by each Schwann cell. Delicate cytoplasmic extensions of fibroblasts F can be seen in the endoneurium.
At high magnification in micrograph (c), part of the cytoplasm of a Schwann cell S is shown ensheathing several axons A; axons are readily identified by their content of smooth endoplasmic reticulum and microtubules, seen in cross-section. Several mesaxons M can be seen. The external surface of the Schwann cell is bounded by an external lamina L, equivalent to lamina densa in epithelia.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine
