Fig. 3.1
Superficial urothelial (umbrella) cells. (a) The cytoplasm of large superficial cells is very frothy and abundant resulting in a low N/C ratio. Nuclei have pale finely granular chromatin. Nucleoli can be prominent, but don’t reflect any abnormality. Multinucleation is common, especially in instrumented samples (Washing, TP, medium mag). (b) In addition to superficial (umbrella) cells, clusters of smaller cells are seen (arrows). The nuclei are darker and slightly smaller than in superficial cells but the nuclear shapes are round, nuclear membranes are smooth, and architecture is uniform. N/C ratios are high due to the small amount of cytoplasm of each cell (Washing, TP, medium mag). (c) These true tissue fragments (TTF) clearly illustrate the image of “umbrella” cells. By definition, they are the most superficial cells in the bladder, creating an “Umbrella” over all other urothelial cells. Their nuclear and cytoplasmic character is the same as other superficial cells, but additionally, they possess a thickened cytoplasmic edge that doesn’t go all around the cell. This constitutes the asymmetric unit membrane, providing a barrier between the toxic urine and the blood (Washing, CS, medium mag)
Superficial cells are large, shaped like the canopy of an umbrella, with rounded (convex) luminal surfaces and scalloped (concaved) borders onto which the underlying intermediate cells are sometimes attached. The cytoplasm is abundant and vacuolated or foamy, not to be mistaken as koilocytes. They are often bi- or multinucleated, or may contain a single large nucleus. The nuclei are centrally located, round to oval, with smooth nuclear membranes. The chromatin is fine and an occasionally prominent chromocenter/nucleolus is present. Characteristically, the N/C ratio is low.
In contrast, intermediate urothelial cells have nuclei with basically the same size and character as superficial cells, but have less cytoplasm, imparting a higher N/C ratio (Fig. 3.2). Since they are less mature than superficial cells, their nuclear chromatin may be a bit coarser than the ubiquitous superficial cells, but thin, even nuclear membranes and uniform chromatin distribution will support their totally benign condition. Cytoplasm will not be as vacuolated as superficial cells, but is not completely opaque (homogeneous), the latter feature being cited as a characteristic of low-grade urothelial neoplasms [3–6].
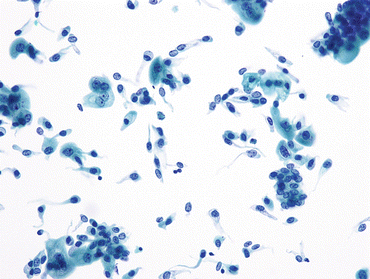

Fig. 3.2
Intermediate urothelial cells. The intermediate layer of urothelium, immediately underneath the umbrella cells, is easily dissociated into single cells. These often have cells with cytoplasmic (cercariaform) tails (All of the features are normal, and described in Fig. 3.1b.) (Washing, TP, medium mag.)
Although superficial cells can sometimes appear very “atypical” by virtue of enlarged nuclei and multiple nucleoli, they are recognized as benign/reactive by their low N/C ratio, characteristic scalloped edges, vacuolated cytoplasm, and smooth nuclear membranes (Fig. 3.3a, b). Even the most “atypical” umbrella cells do not justify the diagnosis of atypia in urine cytology. What is of significance, however, is the fact that umbrella cells may contain abnormal amounts of DNA [7] and may be a potential pitfall in any ancillary tests that are based upon DNA ploidy, including fluorescence in situ hybridization (FISH) [8–10]. A reason for the “reactive” changes should be sought, either from clinical history, or from the sample itself, e.g., neutrophils, fungi, calculi.
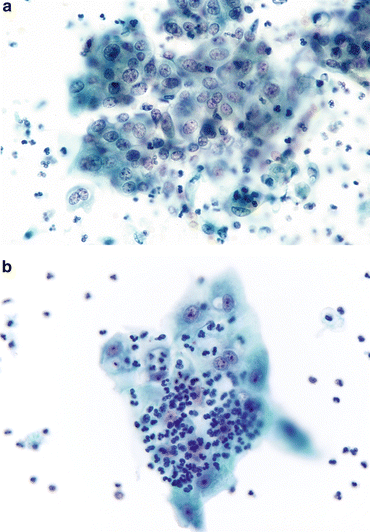

Fig. 3.3
Reactive umbrella cells. (a) Most inflamed epithelial cells demonstrate changes, especially in the nuclei. Nucleoli may become prominent, but nuclear chromatin will remain finely granular and shapes will remain round. The cytoplasm retains its transparency. Neutrophils will ordinarily be the inflammatory cells, but lymphocytes may be present if a chronic process is ongoing (Washing, CS, medium mag.). (b) Changes consistent with epithelial repair are identical with those in all other body sites. The epithelium appears stretched (like Turkish taffy) but all cells stay connected, retaining their intercellular connections. Inflammatory cells pepper the TTF (Washing, TP, medium mag.)
Squamous Epithelial Cells, Both Superficial and Intermediate
Both men and women can be expected to have benign squamous cells (Fig. 3.4) in their urine, although they are much more common in women. In voided urine from a woman, the origin is usually the urethra but may be a contaminant from the vagina or perineum. If the sample is instrumented, then the origin is either the urethra or the bladder trigone in both men and women. Of note, chronic irritation, particularly due to stones, can cause squamous metaplasia leading to presence of squamous cells in instrumented urines. Squamous cells can arise from the trigone secondary to hormonal influence and resemble changes seen in the vaginal epithelium. Only if there are tangible abnormal nuclear changes in the squamous cells should the sample be placed into the atypical category. In younger women it can indicate contamination from the cervix or vagina but, in an older population, it may point to an underlying HGUC with squamous differentiation.
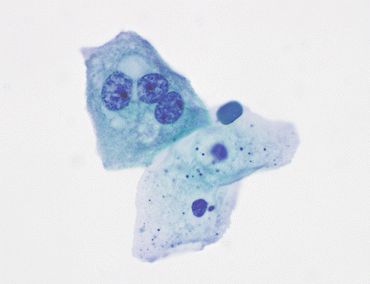

Fig. 3.4
Benign squamous cells. Two benign squamous cells line up below an umbrella cell with three nuclei. The presence of squamous cells in voided urine may come from external genitalia, including the vagina. In a catheterized patient, their origin is usually in an area of metaplasia in the lining of the bladder between the ureteral orifices and the urethra, the trigone (VU, TP, medium mag.)
Glandular Cells
In voided urine from women, benign glandular cells (Fig. 3.5a, b) may be derived from the uterine cervix or corpus and are usually few and degenerated. Endometrial cells may appear in voided urine specimens in women of all ages. Endometrial-type cells are characterized as cohesive, three-dimensional aggregates of small glandular cells with scant cytoplasm, slightly irregular nuclei with vesicular fine chromatin, and visible small nucleoli. A few clusters in a menstruating woman are of no consequence. However, when the patient is menopausal, and endometrial cells in her voided urine are present, this is an alert to her clinicians (see Chap. 8).
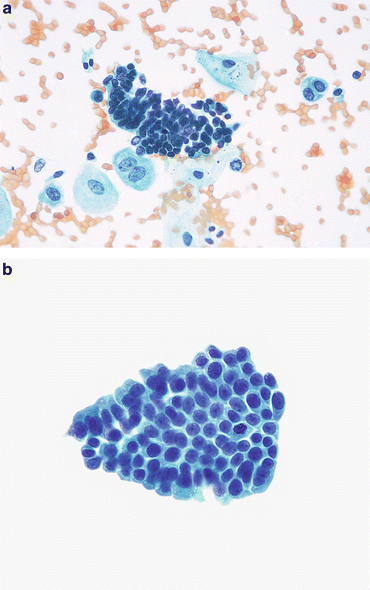

Fig. 3.5
Benign glandular cells—endometriosis, Mullerianosis. (a) Glandular cells in a urinary tract specimen may be native to the urinary collecting system, or external to it. The larger cells are urothelial or squamous. The cluster of small dark cells originated in an endometriosis of the ureter. The patient was presenting with hematuria and pain coincident with the patient’s menstrual periods (Ureteral brushing, CS, high mag.). (b) Glandular cells from a focus of Mullerianosis are not distinguishable from other glandular cells in the urinary bladder. This very rare lesion is related to endometriosis, both considered a metaplasia. Their origin must be biopsy proven correlating the morphology with the location deep in the bladder wall (Bladder washing, SP, medium mag.)
In urine collected by bladder instrumentation, glandular type cells from the urinary tract will be well preserved, with small nuclei and vacuolated cytoplasm. Most often they are true tissue fragments, but also can be seen as single cells. Their origins are many: glandular epithelium in the dome of the bladder (urachal remnant) or trigone are developmental, not metaplasias. Endometriosis may involve the urinary tract, and present an unexpected cellular picture of very small hyperchromatic cells, either in voided urine or brushings from the ureter (see Fig. 3.5a); cells from Mullerian rests (Mullerianosis) are also native, albeit rare (see Fig. 3.5b). Conversely, cystitis cystica/glandularis (Fig. 3.6a–c) is a metaplasia of the urothelium resulting from chronic inflammation.
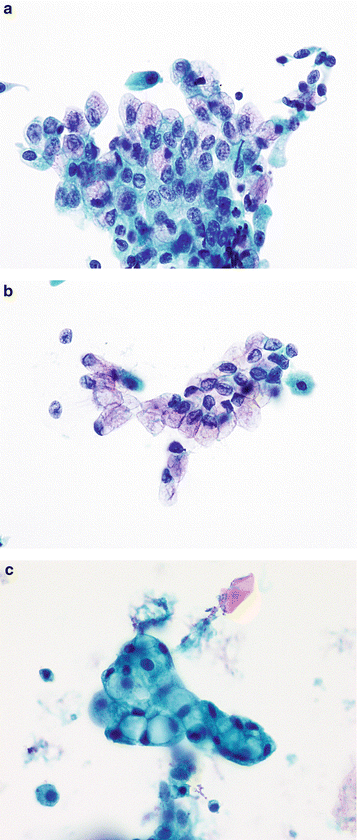

Fig. 3.6
Cystitis cystica/glandularis. (a) Glandular cells from the lining of the bladder can originate from a focus native to the urothelium, metaplasia from an inflammatory focus (cystitis cystica/glandularis), or from a glandular neoplasm either primary or secondary. Unless the cytomorphology suggests a neoplasm, all such TTFs or cell groups are considered benign (Washing, TP, high mag.). (b) Cystitis cystica may appear as in Fig. 3.6a or as a single layer of glandular cells. They closely resemble endocervical cells, and could be from a case of endocervicosis if the tumor was also in the muscular wall of the bladder or ureter. This mucosal strip was from a focus of cystitis cystica (Washing, TP, high mag.). (c) Another tight glandular group, a TTF, demonstrates nuclear compression by relatively large cytoplasmic vacuoles. Regardless of their origin, these cells fulfill the criteria of benignity, making the diagnosis of “Negative” appropriate (Washing, TP, high mag.)
Renal tubular epithelial cells (Fig. 3.7 a–c) will usually appear degenerated and resemble histiocytes (see Fig. 3.7a), especially if single. Those from the proximal and distal convoluted tubules may appear quite columnar, especially in a true tissue fragment (see Fig. 3.7b) or a cast, whereas those from the loop of Henle will still look like histiocytes, even when seen as an intact cast (see Fig. 3.7c).
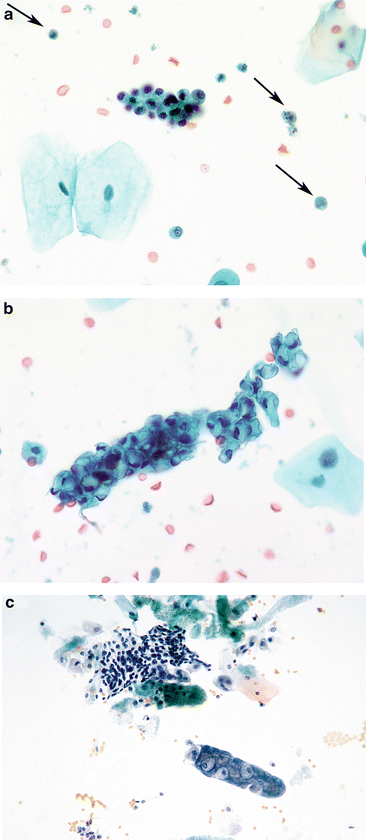

Fig. 3.7
Renal tubular epithelial cells (RTEC). (a) RTEC—histiocytic type: RTEC can be very small, the size of histiocytes. They may have vacuolated cytoplasm in varying amounts and may occur singly (arrows) in urinary samples. When aggregated as this group, a cast should be considered, implying renal disease. There are usually variable amounts of cellular degeneration (Voided, SP, high mag.). (b) RTEC—glandular. This large TTF of RTEC is unusual in size, and represents a tubular cast. The renal tubular cells vary from small with scant cytoplasm to larger and vacuolated. The patient was in renal failure (Voided, SP, high mag.). (c) RTEC—cast. RTEC within this cast demonstrate small nuclei with relatively abundant cytoplasm. The material supporting the RTEC in the cast is protein. The patient was in renal failure (Voided, CS, high mag.)
Benign Urothelial Tissue Fragments
Benign Urothelial Tissue Fragments in Voided Urine
Although most cytologists regard urothelial tissue fragments in voided urines (UTF) as abnormal, a recent review of such samples has found them commonplace and benign [11]. Causes of BUTF (Fig. 3.8a) in voided urines are multifold, and include: prostate/rectal manipulation prior to collection of the sample, jogging, abdominal palpation, etc. Most often BUTF are of no clinical importance. Table 3.1 demonstrates the results of slide review of cases that contained BUTF and atypical urothelial tissue fragments (AUTF) with their follow-up. The results are impressive, and support the fact that cytomorphology and architecture are the most important criteria to suspect LGUN [12] when interpreting the sample; however, the presence of AUTF is more commonly present in tissue-confirmed LGUC than BUTF.
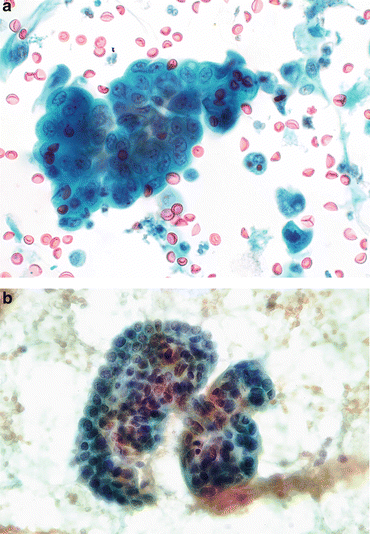

Fig. 3.8
Benign urothelial tissue fragment (BUTF). (a) Voided. BUTF can be seen in voided urines, and should not mandate a diagnosis of “atypical”. In this fragment, nuclei are uniform in size and shape, evenly spaced, and with finely granular chromatin (Voided, SP, high mag.). (b) Instrumented from renal pelvis. Cell fragments from the renal pelvis should be cautiously considered. In this case, the diagnosis rendered was “suspicious for low-grade neoplasm”. The excision of the kidney revealed only urothelial hyperplasia overlying a subepithelial hemangioma. Retrospective review recognized the uniform nuclear size and round shape. The resemblance to a papillary lesion was no doubt the result of instrumentation (Renal pelvic washing, CS, high mag.)
Follow-up diagnosis | No. (%) | No. (%) |
|---|---|---|
BUTF | AUTF | |
Histopathology | 29 (10.6) | 24 (14.1) |
Benign | 17 (6.2) | 6 (3.5) |
Urothelial neoplasia | 12 (4.4) | 17 (10.0) |
PUNLMP | 1 (0.4) | 0 (0.0) |
LGUC | 9 (3.3) | 2 (1.2) |
HGUC | 2 (0.7) | 15 (8.8) |
Cytopathology | 45 (16.4) | 25 (14.7) |
Benign/AUC | ||
Urolithiasis (Clinical/radiologic/gross) | 45 (16.4) | 49 (28.8) |
No follow-up | 25 (9.1) | 10 (5.9) |
Benign Urothelial Tissue Fragments in Instrumented Urine
Instrumented urine specimens are often cellular, consisting of numerous benign-appearing cells arranged in groups that resemble papillary clusters with smooth (community) borders, and that lack fibrovascular cores (Fig. 3.8b). These are defined as “true tissue fragments” and need to be considered by the cytologist as to their category placement. This will depend upon their nuclear and architectural details. If all the criteria of benign urothelial cells, as outlined above, are present then the call is BUTF and the sample is considered NHGUC, unless other criteria of atypia, suspicious or HGUC are present in the specimen, or if evidence of another significant lesion, e.g., LGUN, is seen [13].
The causes of such BUTF may be neoplasm, recent or concurrent instrumentation, or most commonly, lithiasis of the urinary tract, usually in the renal pelvis (See discussion under section “Risk of Malignancy”).
Clusters, Groups, or Sheets of Urothelial Cells
Clusters/groups or sheets of benign urothelial cells (Fig. 3.9a,b), visually different from BUTF (see Fig. 3.8), are commonly found in benign samples and have no significance, so long as the nuclei bear benign characteristics.
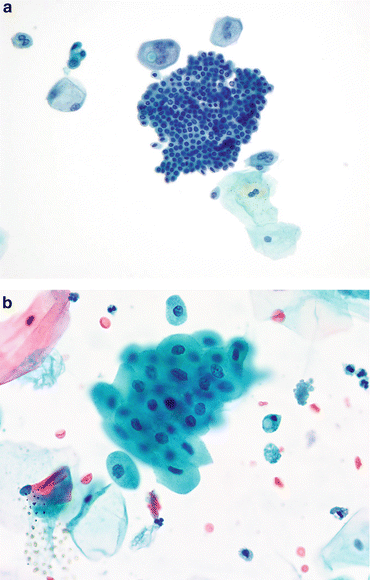

Fig. 3.9
Clusters or sheets of urothelial cells. (a) If sheets or clusters have “windows” they cannot be considered TTFs, BUTFs, or AUTFs if they have no atypia. This sheet is essentially a monolayer of uniform cells with round nuclei and uniformly pale chromatin. N/C ratios are high, a reflection of the intermediate position of these cells in the urothelial layer (Washing, TP, medium mag.). (b) Urothelial cells may undergo squamous metaplasia, indicated by their opaque cytoplasm and sharp cellular borders. Nuclei are small. All of these features indicate a cell group that may be a BUTF, and is certainly benign (Voided, SP, high mag.)
Explanatory Note: Instrumented urine specimens include any specimen that was obtained by any instrument or whenever force was applied to dislodge individual cells from the lining urothelium. These include catheterized urines, bladder washings, brushings, and upper urinary tract urine specimens obtained by urinary catheterization. In addition, any immediately post-cystoscopy urine specimen is also considered instrumented. In general, these specimens are cellular, and the presence of BUTF in instrumented urine specimens is a normal finding; therefore, we should avoid the term “atypia” in this context in these types of specimens so long as the nuclear and architectural features of the UTF do not warrant another diagnosis.
Three-Dimensional Urothelial Tissue Fragments with Nephrolithiasis
Voided urine specimens from patients who may present with hematuria and/or filling defect on imaging studies are often cellular and consist of three-dimensional urothelial fragments composed of cells that may exhibit significant pleomorphism (Fig. 3.10a, b). These three-dimensional urothelial fragments display smooth (community) borders, and cytoplasmic collars/collarets, i.e., a rim of cytoplasm surrounding the nuclei as in BUTF. If a cause of the atypia is found, such as a stone (Fig. 3.10c), then the diagnostic category should be NHGUC.
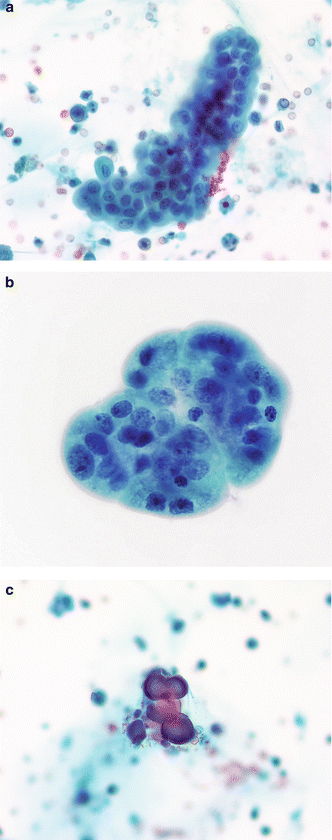

Fig. 3.10




Urothelium with nephrolithiasis—three-dimensional fragments. (a) Kidney and bladder stones can cause serious changes in the urothelium, sometimes resembling neoplasms. Careful examination of the cells in a three-dimensional TTF is critical to an accurate diagnosis. These cells have round nuclei which are evenly spaced. Chromatin is finely granular and nucleoli are inconspicuous. A renal calculus was discovered on imaging studies and from the clinical history (Voided, SP, moderate mag.). (b) A BUTF in a voided urine may be the result of numerous causes. In this patient, nephrolithiasis was the reason. Cellular changes are mild when compared to those in the photos to follow (Chap. 4). The absence of any fibrovascular stalk eliminates the diagnosis of a low grade LGUN (Voided, SP, high mag.). (c) Calcific concretions in voided urine may be recovered in patients with history of renal calculi (Voided, SP, high mag.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


