The Hypothalamic-Hypophyseal Tract & Blood Supply
Adenohypophysis (Anterior Pituitary)
Control of Hormone Secretion in the Anterior Pituitary
Neurohypophysis (Posterior Pituitary)
Production of Thyroid Hormone & Its Control
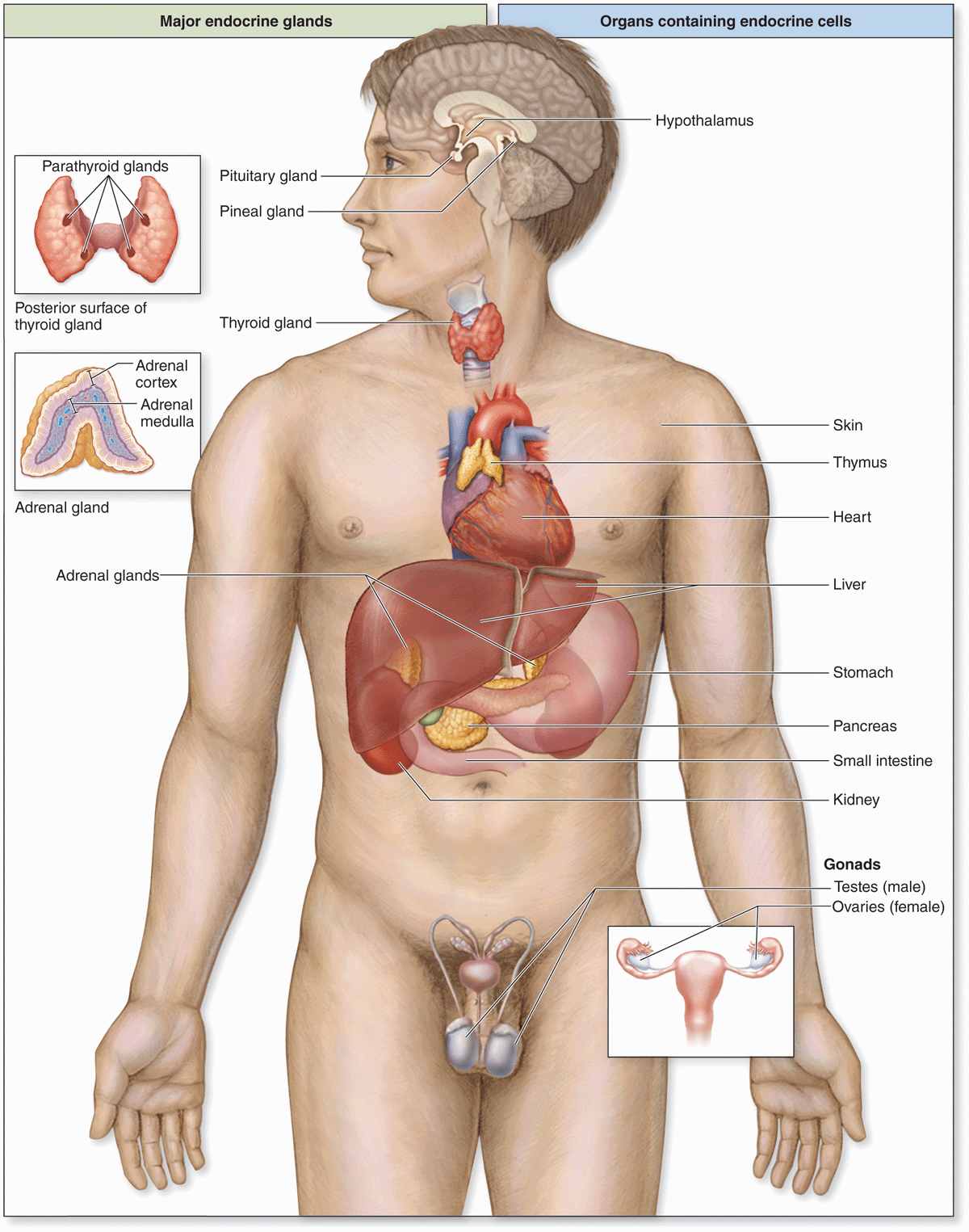
Secretory cells of endocrine glands release their products, signaling molecules called hormones, into the neighboring vascularized compartment for uptake by capillaries and distribution throughout the body. There is no secretory duct as in exocrine glands. Endocrine cells are typically epithelial, at least in origin, and aggregated as cords or clusters. Besides the specialized endocrine glands discussed in this chapter, many other organs specialized for other functions, such as the heart, thymus, gut, kidneys, testis, and ovaries, contain various endocrine cells (Figure 20–1).
FIGURE 20–1 Locations of the major endocrine glands.
Distribution by the circulation allows hormones to act on target cells with receptors for those hormones at a distance from the site of their secretion. As discussed briefly in Chapter 2, other endocrine cells produce hormones that act on target cells only a short distance away. This may involve paracrine secretion, with localized dispersal in interstitial fluid or through short loops of blood vessels, as when gastrin made by pyloric G cells reaches target cells in the fundic glands, or juxtacrine secretion, in which a signaling molecule remains on the secreting cell’s surface or adjacent extracellular matrix and affects target cells when the cells make contact. Juxtacrine signaling is particularly important in embryonic and regenerative tissue interactions. In autocrine secretion, cells may produce molecules that act on themselves or on cells of the same type. For example, insulin-like growth factor (IGF) produced by several cell types may act on the same cells that produced it. Endocrine glands are often also target organs for other hormones that can establish a feedback mechanism to control hormone secretion and keep blood hormonal levels within strict limits.
Hormones, like neurotransmitters, are frequently hydrophilic molecules such as proteins, glycoproteins, peptides, or modified amino acids with receptors on the surface of target cells. Alternatively, hydrophobic steroid and thyroid hormones must circulate on transport proteins but can diffuse through the cell membranes and activate cytoplasmic receptors in target cells (see Chapter 2).
PITUITARY GLAND (HYPOPHYSIS)
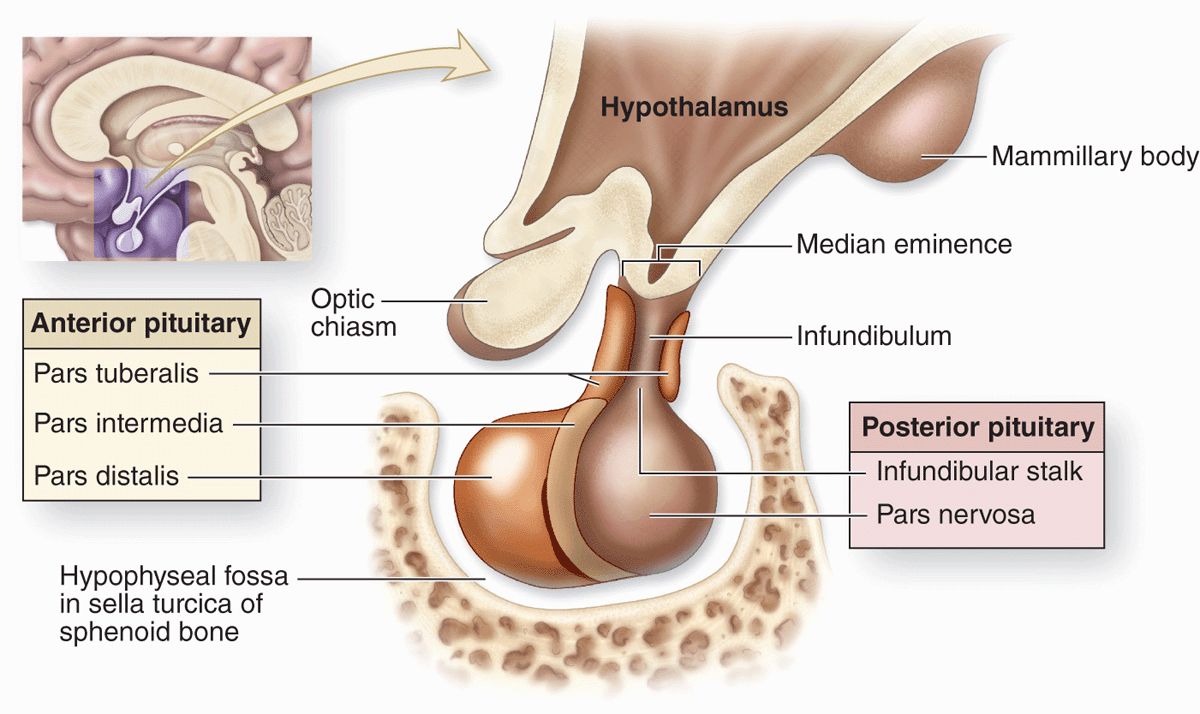
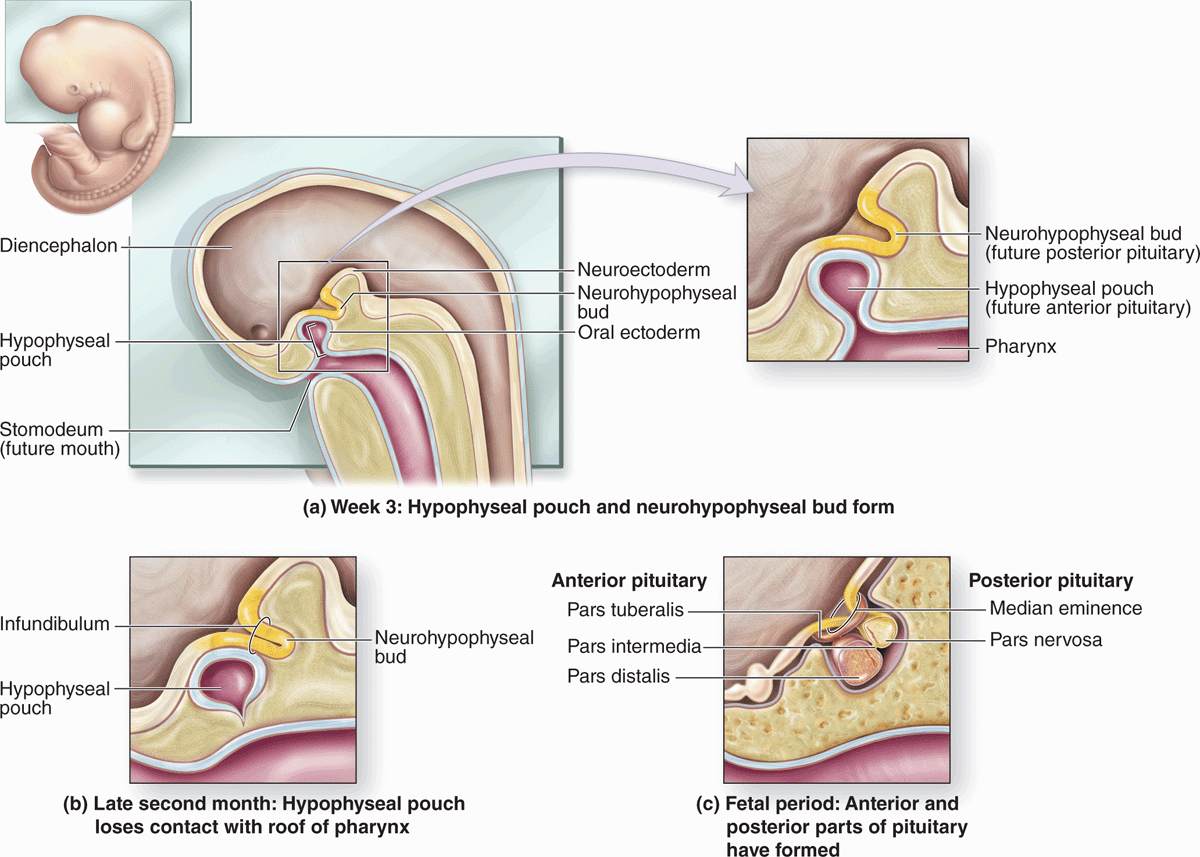
The pituitary gland, or hypophysis (Gr. hypo, under + physis, growth), weighs about 0.5 g in adults and has dimensions of about 10 × 13 × 6 mm. It lies below the brain in a small cavity on the sphenoid bone, the sella turcica (Figure 20–2). The pituitary is formed in the embryo partly from the developing brain and partly from the developing oral cavity (Figure 20–3). The neural component is the neurohypophyseal bud growing down from the floor of the future diencephalon as a stalk (or infundibulum) that remains attached to the brain. The oral component arises as an outpocketing of ectoderm from the roof of the primitive mouth and grows cranially, forming a structure called the hypophyseal (Rathke) pouch. The base of this pouch eventually constricts and separates from the pharynx. Its anterior wall then thickens greatly, reducing the pouch’s lumen to a small fissure (Figure 20–3).
FIGURE 20–2 Pituitary gland.
FIGURE 20–3 Formation of the pituitary gland.
Because of its dual origin, the pituitary actually consists of two glands—the posterior neurohypophysis and the anterior adenohypophysis—united anatomically but with different functions. The neurohypophysis retains many histologic features of brain tissue and consists of a large part, the pars nervosa, and the smaller infundibulum stalk attached to the hypothalamus at the median eminence (Figure 20–2 and 20–4). The adenohypophysis, derived from the oral ectoderm, has three parts: a large pars distalis or anterior lobe; the pars tuberalis, which wraps around the infundibulum; and the thin pars intermedia adjacent to the posterior pars nervosa (Figures 20–2 and 20–4).
FIGURE 20–4 Pituitary gland.
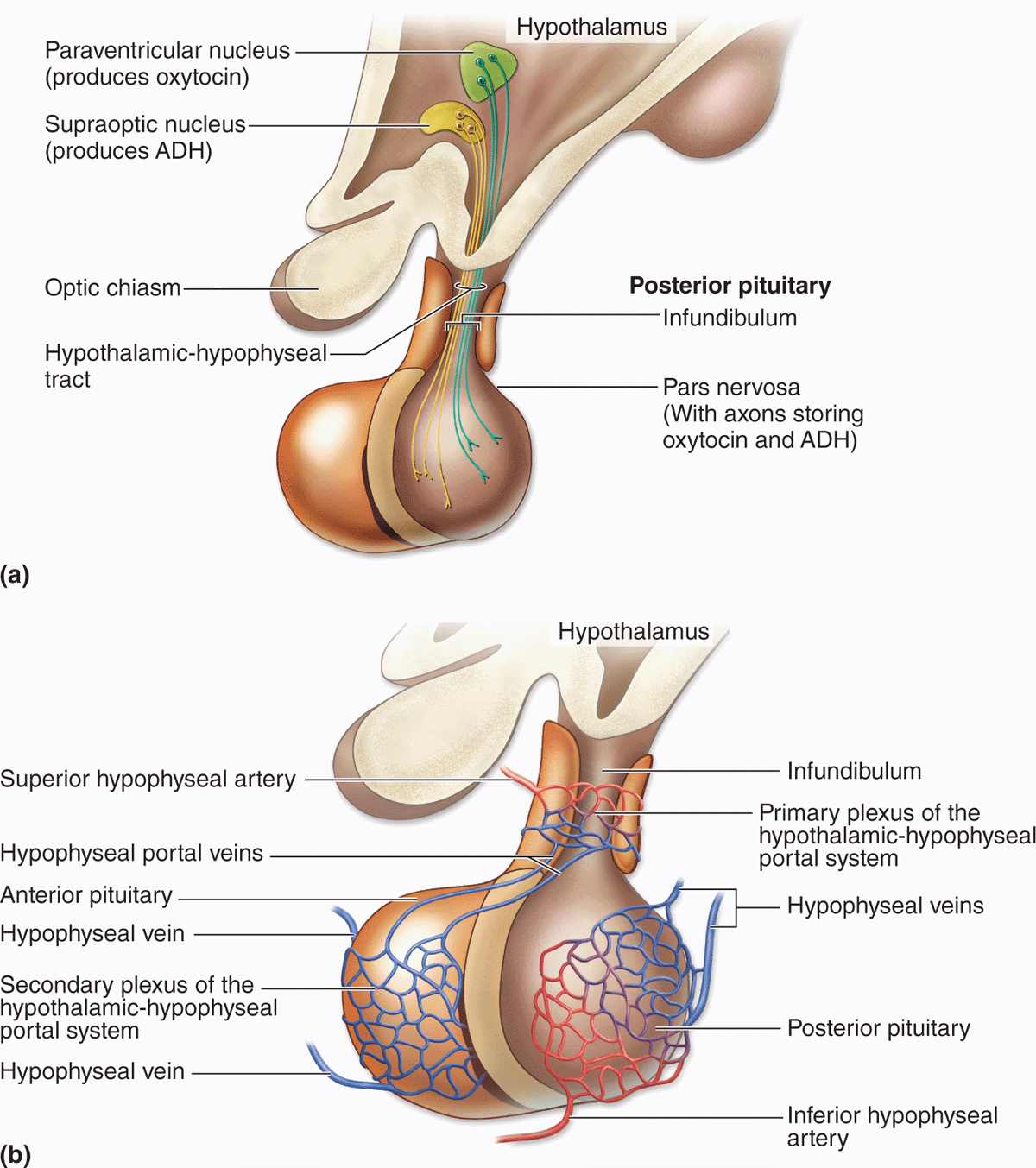
The Hypothalamic-Hypophyseal Tract & Blood Supply
The pituitary gland’s neural connection to the brain and its blood supply are both of key importance for its function (Figures 20–4 and 20–5). Embryologically, anatomically, and functionally, the pituitary gland is connected to the hypothalamus at the base of the brain. In addition to the vascular portal system carrying small regulatory peptides from the hypothalamus to the adenohypophysis, a bundle of axons called the hypothalamic-hypophyseal tract courses into the neurohypophysis from two important hypothalamic nuclei. The peptide hormones ADH (antidiuretic hormone) and oxytocin are synthesized by large neurons in the supraoptic and the paraventricular nuclei, respectively. Both hormones undergo axonal transport and accumulate temporarily in the axons of the hypothalamic-hypophyseal tract before their release and uptake by capillaries branching from the inferior arteries.
FIGURE 20–5 The hypothalamic-hypophyseal tract and portal system.
The blood supply derives from two groups of vessels coming off the internal carotid artery and drained by the hypophyseal vein. The superior hypophyseal arteries supply the median eminence and the infundibular stalk; the inferior hypophyseal arteries provide blood mainly for the neurohypophysis. The superior arteries divide into a primary plexus of fenestrated capillaries that irrigate the stalk and median eminence. These capillaries then rejoin to form venules that branch again as a larger secondary capillary plexus in the adenohypophysis (Figure 20–5). These vessels make up the hypothalamic-hypophyseal portal system that has great importance because it carries neuropeptides from the median eminence the short distance to the adenohypophysis where they either stimulate or inhibit hormone release by the endocrine cells there.
Adenohypophysis (Anterior Pituitary)
The three parts of the adenohypophysis are derived embryonically from the hypophyseal pouch.
Pars Distalis
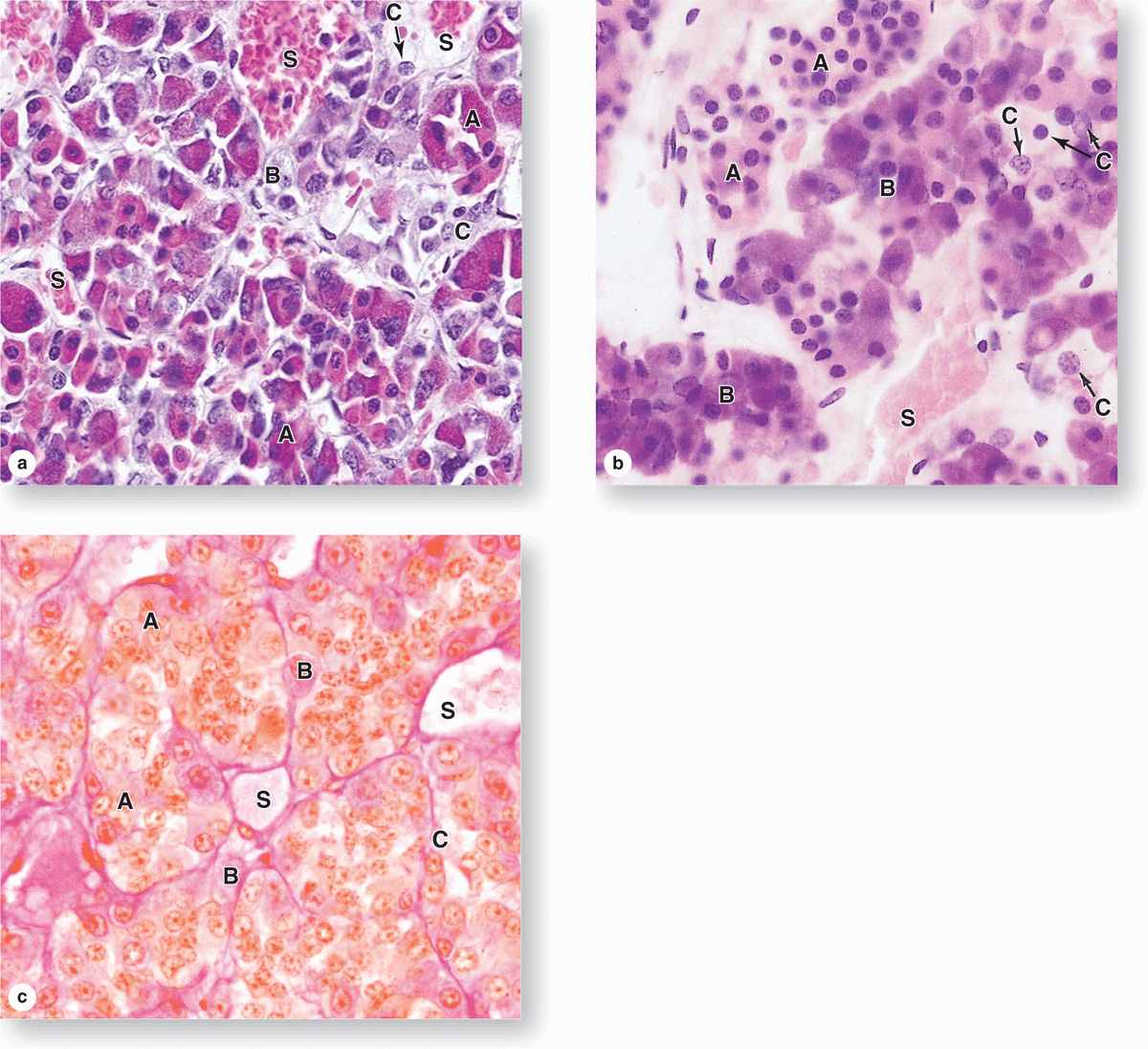
The pars distalis accounts for 75% of the adenohypophysis and has a thin fibrous capsule. The main components are cords of well-stained endocrine cells interspersed with fenestrated capillaries and supporting reticular connective tissue (Figures 20–4 and 20–6). Common stains suggest two broad groups of cells in the pars distalis with different staining affinities: chromophils and chromophobes. Chromophils are secretory cells in which hormone is stored in cytoplasmic granules. They are also called basophils and acidophils, based on their affinities for basic and acidic dyes, respectively (Figure 20–6).
FIGURE 20–6 Pars distalis: Acidophils, basophils, and chromophobes.
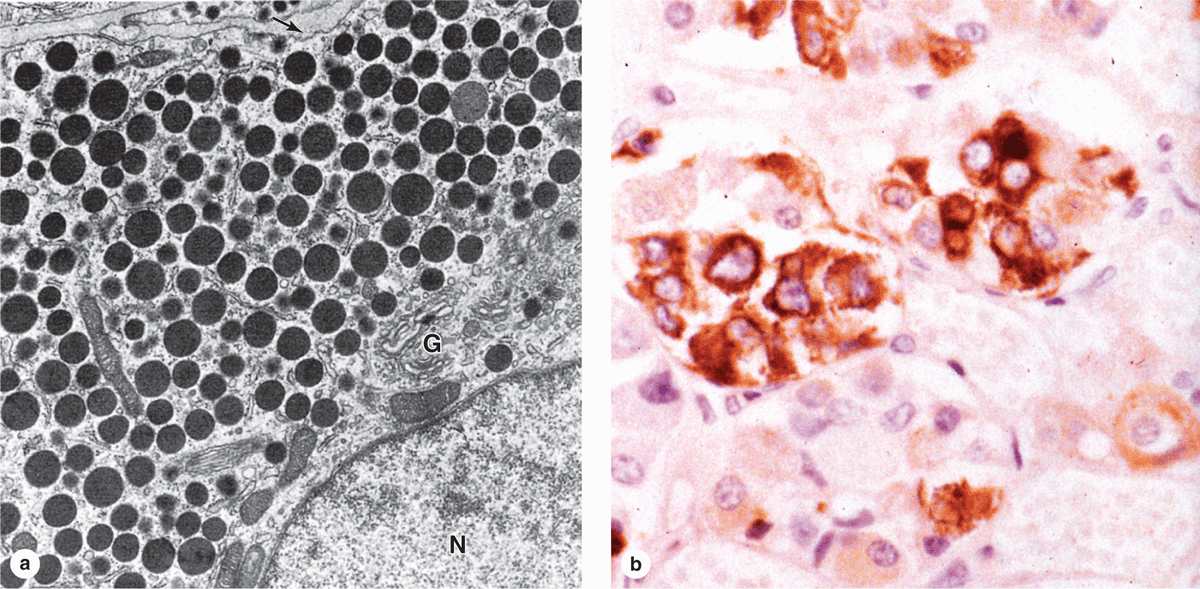
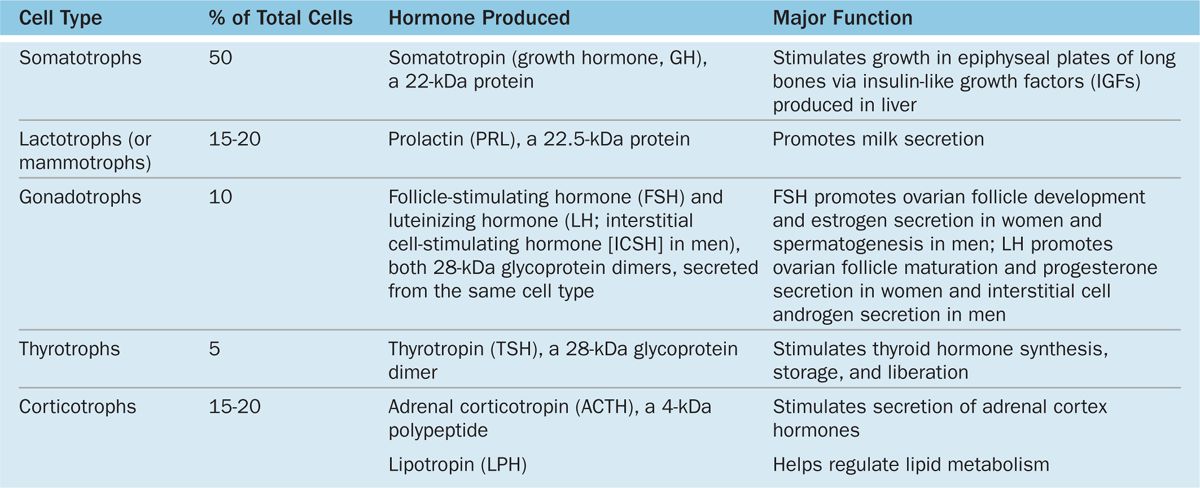
Subtypes of basophilic and acidophilic cells are identified by their granular morphology in the TEM or more easily by immunohistochemistry (Figure 20–7). Specific cells are usually named according to their hormone’s target cells (Table 20–1). Acidophils secrete either growth hormone (somatotropin) or prolactin and are called somatotrophs and lactotrophs (or somatotropic cells and lactotropic cells), respectively. The basophilic cells are the corticotrophs, gonadotrophs, and thyrotrophs, with target cells in the adrenal cortex, gonads, and thyroid gland, respectively. Somatotrophs typically constitute about half the cells of the pars distalis in humans, with thyrotrophs the least abundant.
FIGURE 20–7 Ultrastructure and immunohistochemistry of somatotropic cells.
TABLE 20–1 Major cell types of the anterior pituitary and their major functions.
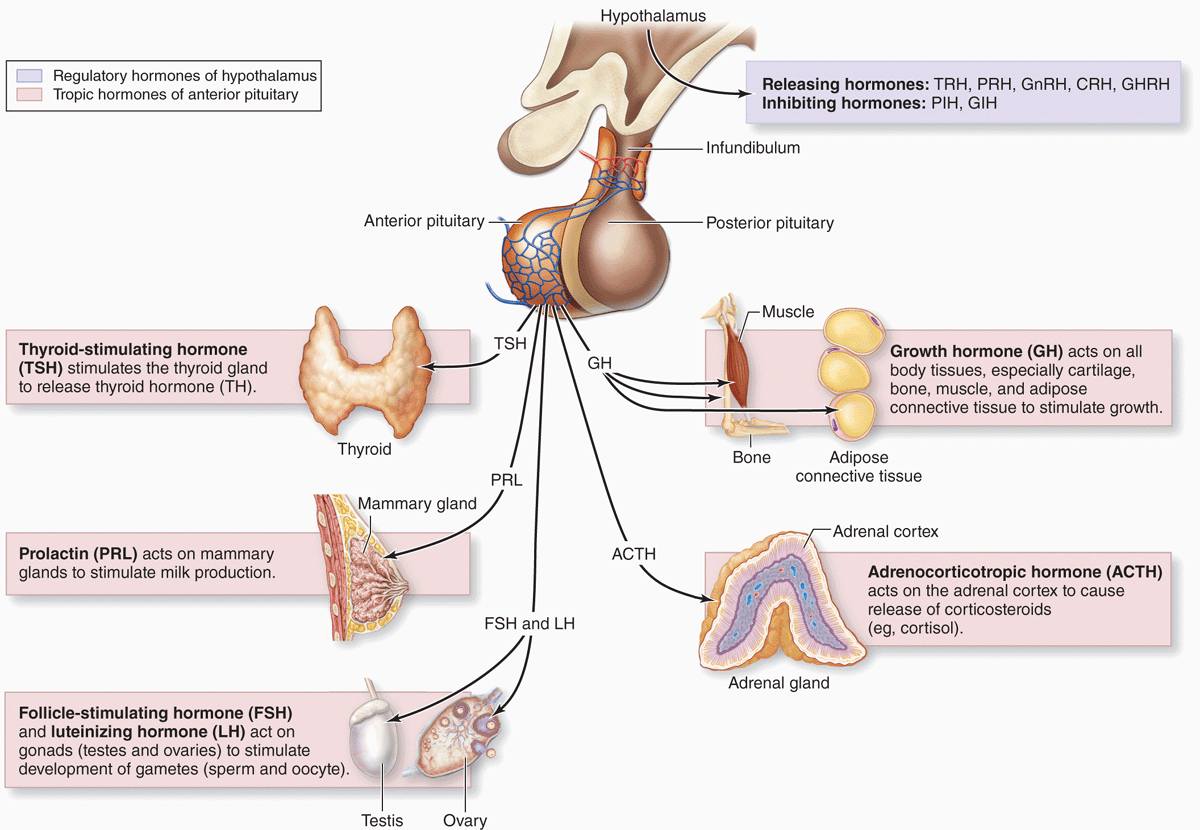
With two exceptions, each type of anterior pituitary cell makes one kind of hormone (see Table 20–1). Gonadotrophs secrete two different glycoproteins: follicle-stimulating hormone (FSH) and luteinizing hormone (LH; called interstitial cell-stimulating hormone [ICSH] in men). The main protein synthesized in corticotrophs is proopiomelanocortin (POMC), which is cleaved posttranslationally into the polypeptide hormones adrenocortical trophic hormone (ACTH) and β-lipotropin (β -LPH). Hormones produced by the pars distalis have widespread functional activities. They regulate almost all other endocrine glands, ovarian function and sperm production, milk production, and the metabolism of muscle, bone, and adipose tissue (see Table 20–1; Figure 20–8).
FIGURE 20–8 Hormones of the pars distalis and their targets.
Chromophobes stain weakly, with few or no secretory granules, and also represent a heterogeneous group, including stem and undifferentiated progenitor cells as well as any degranulated cells present.
Pars Tuberalis
The pars tuberalis is a smaller funnel-shaped region surrounding the infundibulum of the neurohypophysis (Figures 20–2 and 20–4). Most of the cells of the pars tuberalis are gonadotrophs.
Pars Intermedia
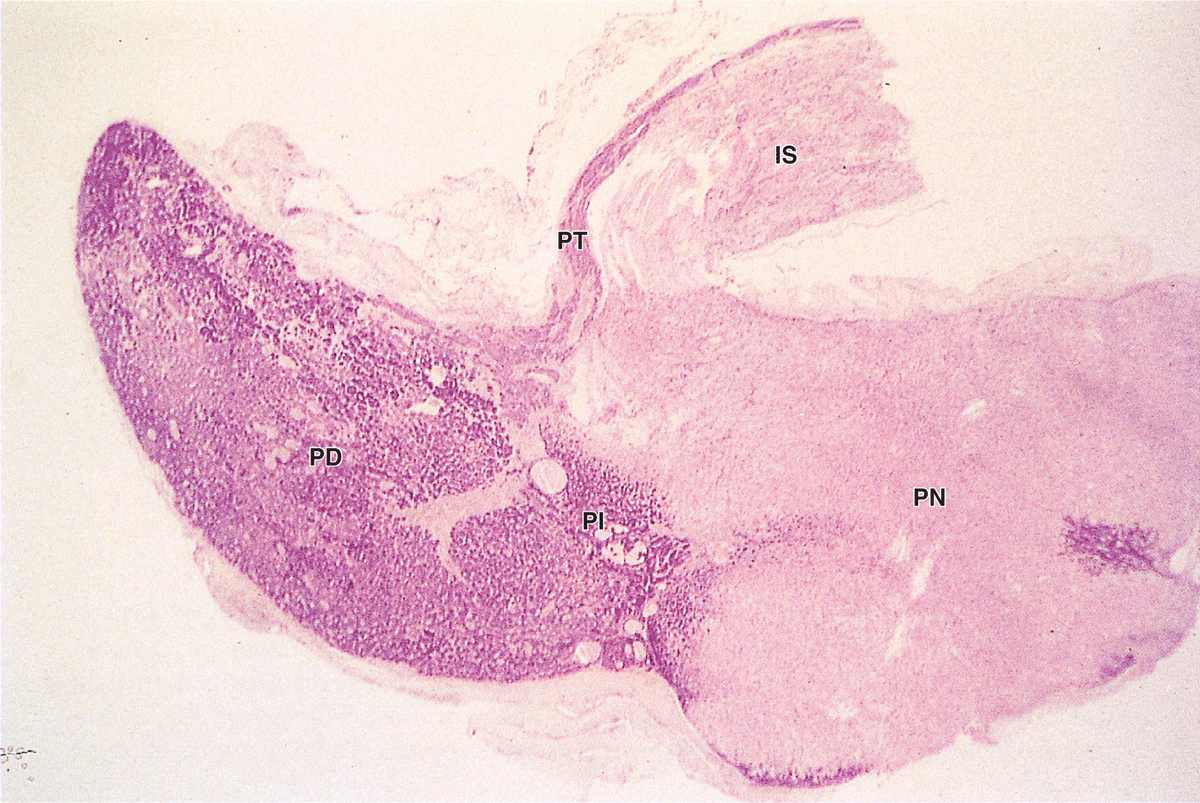
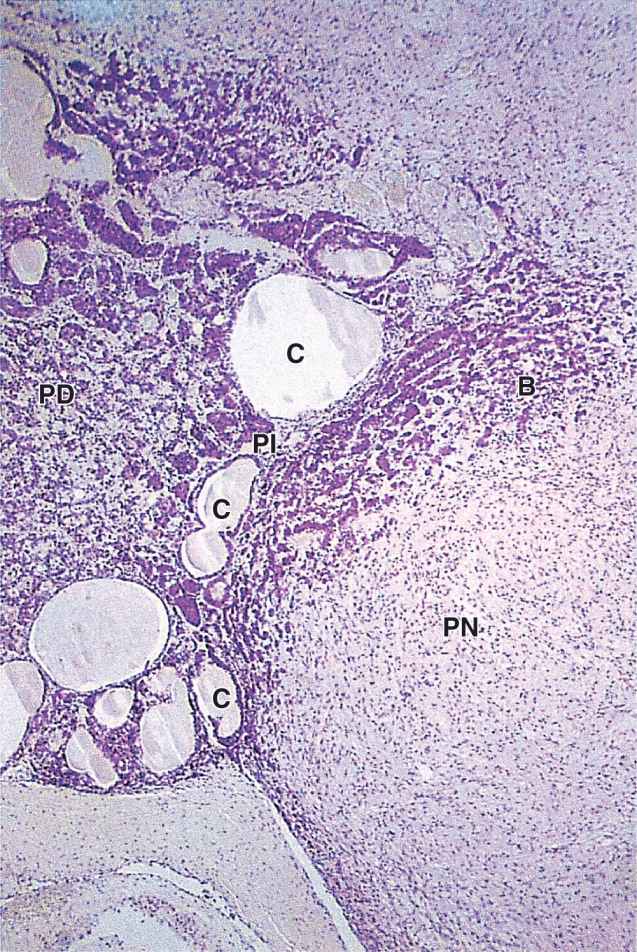
The pars intermedia is a thin zone of basophilic cells between the pars distalis and the pars nervosa of the neurohypophysis, which is often invaded by basophils (Figure 20–9). The pars intermedia develops from the dorsal wall of the hypophyseal pouch and usually contains colloid-filled cysts of various sizes that represent remnants of that structure’s lumen (Figure 20–9). During fetal life cells of this region, like corticotrophs of the pars distalis, express POMC. However, in these cells POMC is cleaved by different proteases to produce smaller peptide hormones, including two forms of melanocyte-stimulating hormone (MSH), γ-LPH, and β-endorphin. MSH increases melanocyte activity, but the overall functional significance of this region remains uncertain, especially in adults.
FIGURE 20–9 Pars intermedia.
Control of Hormone Secretion in the Anterior Pituitary
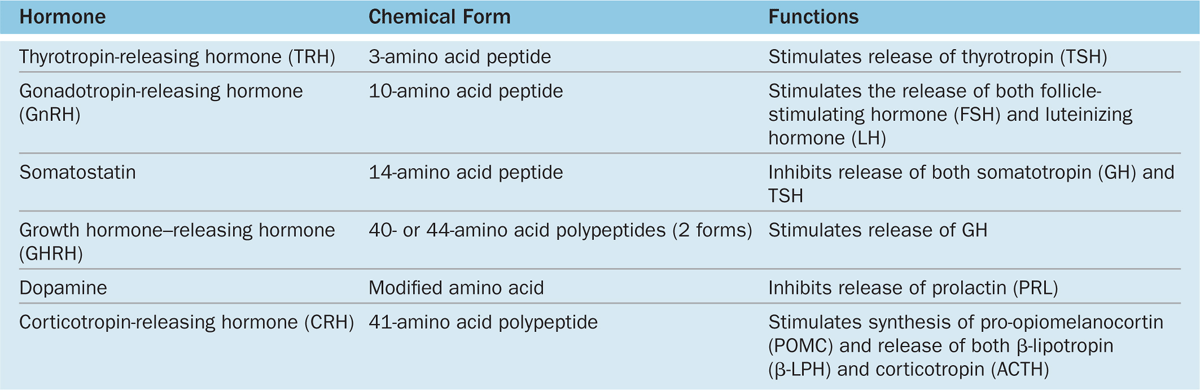
The activities of the cells of the anterior pituitary are controlled primarily by peptide-related hypothalamic hormones produced by small neurons near the third ventricle, discharged from axons in the median eminence, and transported by capillaries of the portal system throughout the anterior pituitary. As shown in Table 20–2, most of these hormones are releasing hormones that stimulate secretion by specific anterior pituitary cells. Two of the hypothalamic factors, however, are inhibiting hormones that block hormone secretion in specific cells of the adenohypophysis (Table 20–2). Because of the strategic position of the hypothalamic neurons and the control they exert on the hypophysis and therefore on many bodily functions, many sensory stimuli coming to the brain, as well as stimuli arising within the CNS, can affect the function of the pituitary gland and then quickly also affect the function of many other organs and tissues.
TABLE 20–2 Hypothalamic hormones regulating cells of the anterior pituitary.
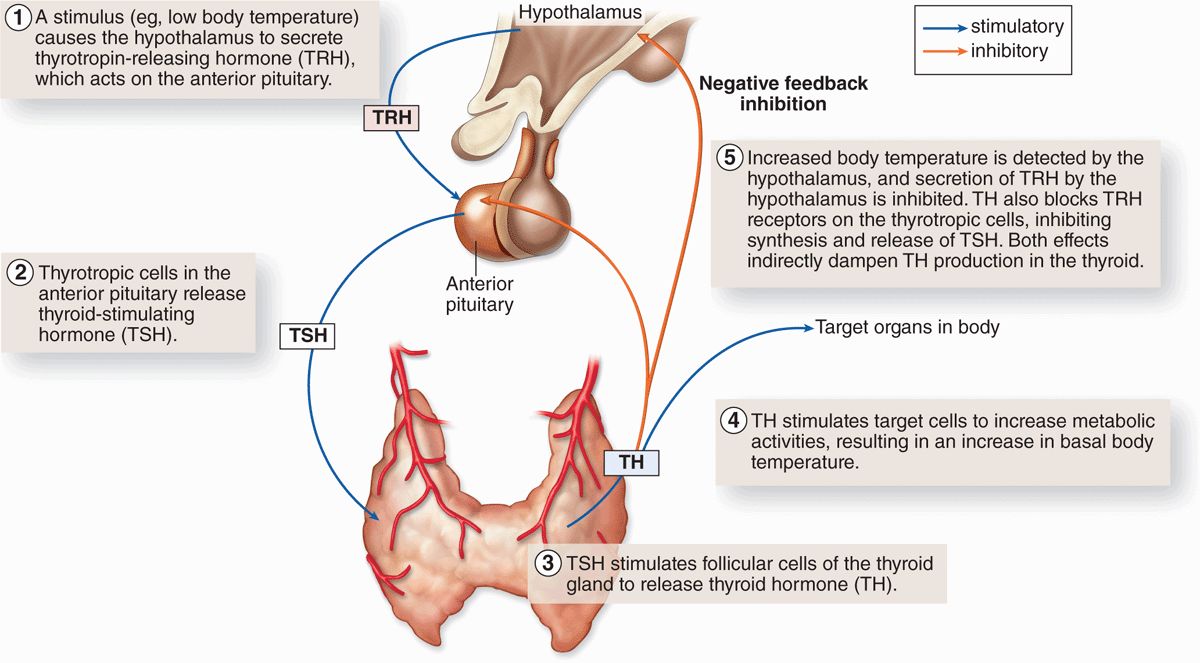
Another mechanism controlling activity of anterior pituitary cells is negative feedback by hormones from the target organs on secretion of the relevant hypothalamic factors and on hormone secretion by the relevant pituitary cells. Figure 20–10 illustrates this mechanism, using the thyroid as an example, and shows the complex chain of events that begins with the action of neural stimuli in the hypothalamus and ends with the effects of hormones from the pituitary’s target organs.
FIGURE 20–10 Negative feedback loops affecting anterior pituitary secretion.
Finally, hormone secretion in the anterior pituitary is affected by other hormones from outside the feedback loop or even outside the major target tissues. Examples include the polypeptide ghrelin produced mainly in the stomach mucosa, which also acts as a releasing hormone for somatotropin secretion, and oxytocin, released in the posterior pituitary during breast-feeding, which increases secretion of prolactin.
All these mechanisms allow the fine tuning of hormone secretion by cells of the anterior pituitary.
Neurohypophysis (Posterior Pituitary)
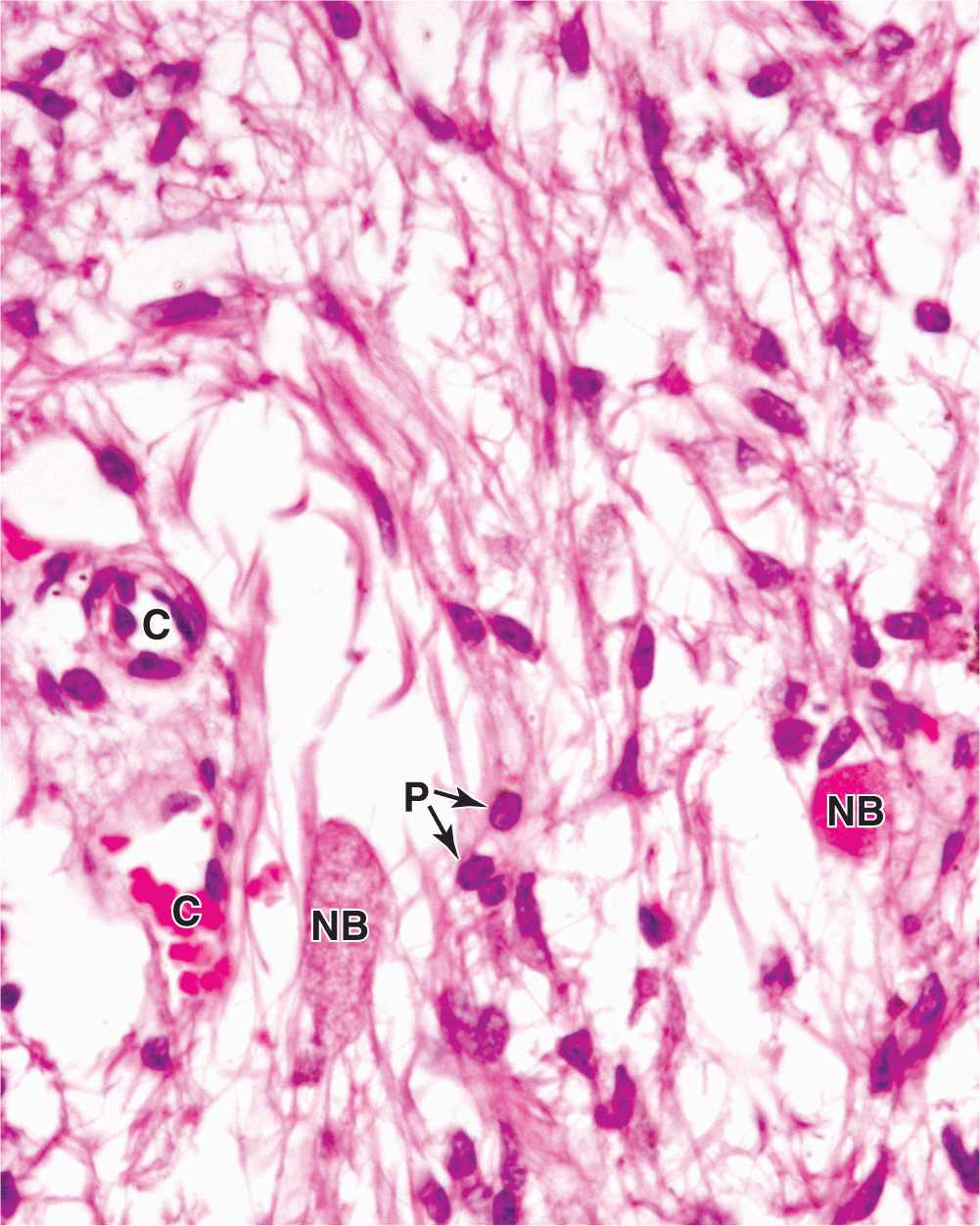
The neurohypophysis consists of the pars nervosa and the infundibular stalk (Figures 20–2 and 20–4) and, unlike the adenohypophysis, does not contain the cells that synthesize its two hormones. It is composed of neural tissue, containing some 100,000 unmyelinated axons of large secretory neurons with cell bodies in the supraoptic and paraventricular nuclei of the hypothalamus (Figure 20–5). Also present are highly branched glial cells called pituicytes that resemble astrocytes and are the most abundant cell type in the posterior pituitary (Figure 20–11).
FIGURE 20–11 Pars nervosa: neurosecretory bodies and pituicytes.
The secretory neurons have all the characteristics of typical neurons, including the ability to conduct an action potential, but have larger-diameter axons and well-developed synthetic components related to the production of the 9-amino acid peptide hormones antidiuretic hormone (ADH)—also called arginine vasopressin—and oxytocin. Transported axonally into the pars nervosa, these hormones accumulate in axonal dilations called neurosecretory bodies or Herring bodies, visible in the light microscope as faintly eosinophilic structures (Figure 20–11). The neurosecretory bodies contain membrane-enclosed granules with either oxytocin or ADH bound to 10-kDa carrier proteins called neurophysin I and II, respectively. The hormone-neurophysin complex is synthesized as a single protein and then cleaved to produce the peptide hormone and its binding protein. Nerve impulses along the axons trigger the release of the peptides from the neurosecretory bodies for uptake by the fenestrated capillaries of the pars nervosa, and the hormones are then distributed to the general circulation. Axons from the supraoptic and paraventricular nuclei mingle in the neurohypophysis but are mainly concerned with ADH and oxytocin secretion, respectively.
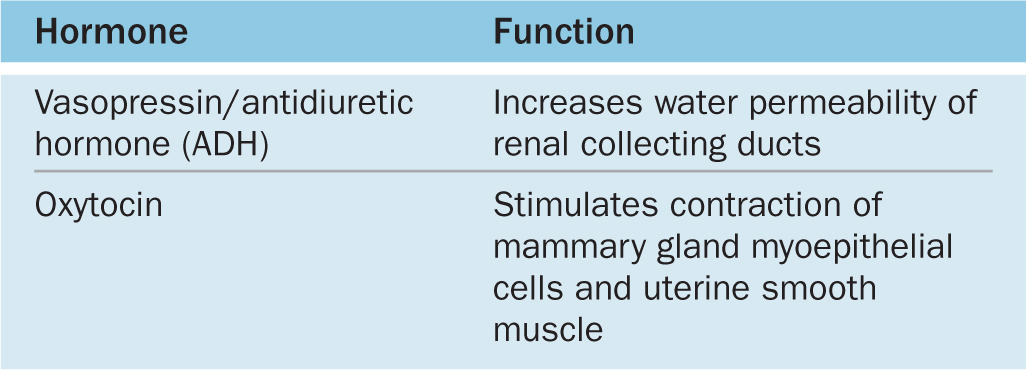
ADH is released in response to increased blood tonicity, sensed by osmoreceptor cells in the hypothalamus, which then stimulate ADH synthesis in supraoptic neurons. ADH increases the permeability of the renal collecting ducts to water (see Chapter 19) so that more water is reabsorbed from the filtrate in these tubules and osmotic balance of body fluids is restored (Table 20–3).
TABLE 20–3 Hormones of the posterior pituitary.
 MEDICAL APPLICATION
MEDICAL APPLICATION


