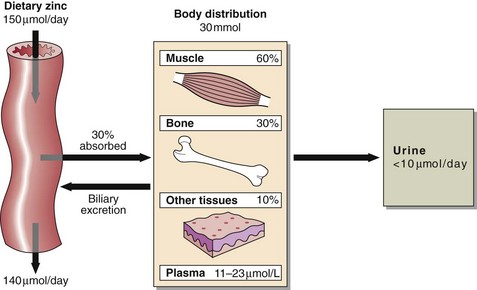
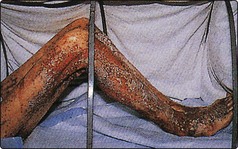
58 Zinc deficiency is a major health problem in the poorer nations of the world. The daily requirement, which varies with age, sex and during pregnancy is about 150 µmol (10 mg) per day (Fig 58.1). Zinc is present in all protein-rich foods and approximately 30% is absorbed. Phytates are indigestible in man, bind calcium, iron and zinc, and reduce their absorption. In the liver, zinc is incorporated into metalloenzymes, while in blood the majority of the zinc is contained in the erythrocytes. In plasma, 90% of zinc is bound to albumin and 10% to α2-macroglobulin. Zinc reserves in the body are small and are located mainly in muscle and bone. Zinc is excreted in urine, in bile, in pancreatic fluid and in milk in lactating mothers. In children, the rate of growth during rehabilitation from famine has been clearly related to the dietary supply of bioavailable zinc. Zinc deficiency is known to occur in patients on intraveneous nutrition and causes a characteristic skin rash (Fig 58.2) and hair loss. Wound breakdown and delayed healing are other complications. Acrodermatitis enteropathica, a rare inherited disorder of zinc metabolism, manifests itself in infancy as skin rash. Untreated, the prognosis is poor, but oral zinc therapy leads to complete remission. Cadmium displaces zinc from metalloproteins, and zinc deficiency can be a consequence of chronic cadmium poisoning.
Zinc and copper
Zinc
Zinc physiology
Zinc deficiency
Zinc and copper