INTRODUCTION
The organisms discussed in this chapter are short, pleomorphic Gram-negative rods that can exhibit bipolar staining. They are catalase positive, oxidase negative, and microaerophilic or facultatively anaerobic. Most have animals as their natural hosts, but they can produce serious disease in humans.
The genus Yersinia includes Yersinia pestis, the cause of plague; Yersinia enterocolitica, an important cause of human diarrheal disease; and several others considered nonpathogenic for humans. Pasteurella are primarily animal pathogens but Pasteurella multocida can also produce human disease.
YERSINIA PESTIS AND PLAGUE
Plague is an infection of wild rodents transmitted from one rodent to another and occasionally from rodents to humans by the bites of fleas. Serious infection often results, which in previous centuries produced pandemics of “black death” with millions of fatalities. The ability of this organism to be transmitted by aerosol and the severity and high mortality associated with pneumonic plague make Y pestis a potential biological weapon.
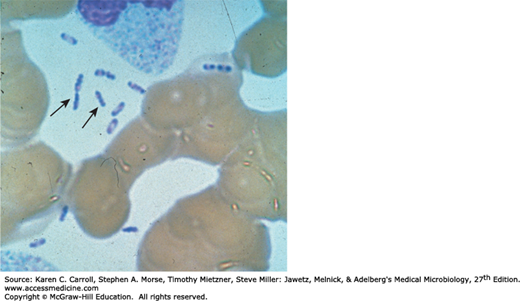
Y pestis is a Gram-negative rod that exhibits striking bipolar staining with special stains such as Wright, Giemsa, Wayson, or methylene blue (Figure 19-1). It is nonmotile. It grows as a facultative anaerobe on many bacteriologic media. Growth is more rapid in media containing blood or tissue fluids and fastest at 30°C. In cultures on blood agar at 37°C, colonies may be very small at 24 hours. A virulent inoculum, derived from infected tissue, produces gray and viscous colonies, but after passage in the laboratory, the colonies become irregular and rough. The organism has little biochemical activity, and this is somewhat variable.
All yersiniae possess lipopolysaccharides that have endotoxic activity when released. Y pestis and Y enterocolitica also produce antigens and toxins that act as virulence factors. They have type III secretion systems that consist of a membrane-spanning complex that allows the bacteria to inject proteins directly into cytoplasm of the host cells. The virulent yersiniae produce V and W antigens, which are encoded by genes on a plasmid of approximately 70 kb. This is essential for virulence; the V and W antigens yield the requirement for calcium for growth at 37°C. Compared with the other pathogenic yersiniae, Y pestis has gained additional plasmids. pPCP1 is a 9.5-kb plasmid that contains genes that yield plasminogen-activating protease that has temperature-dependent coagulase activity (20°–28°C, the temperature of the flea) and fibrinolytic activity (35°–37°C, the temperature of the host). This factor is involved in dissemination of the organism from the flea bite injection site. The pFra/pMT plasmid (80–101 kb) encodes the capsular protein (fraction F1) that is produced mainly at 37°C and confers antiphagocytic properties. In addition, this plasmid contains genes that encode phospholipase D, which is required for organism survival in the flea midgut.
Y pestis and Y enterocolitica have a pathogenicity island (PAI) that encodes for an iron-scavenging siderophore (see Chapter 9), yersiniabactin.
When a flea feeds on a rodent infected with Y pestis, the ingested organisms multiply in the gut of the flea and, helped by the coagulase, block its proventriculus so that no food can pass through. Subsequently, the “blocked” and hungry flea bites ferociously, and the aspirated blood, contaminated with Y pestis from the flea, is regurgitated into the bite wound. The inoculated organisms may be phagocytosed by polymorphonuclear cells and macrophages. The Y pestis organisms are killed by the polymorphonuclear cells but multiply in the macrophages; because the bacteria are multiplying at 37°C, they produce the antiphagocytic protein and subsequently are able to resist phagocytosis. The pathogens rapidly reach the lymphatics, and an intense hemorrhagic inflammation develops in the enlarged lymph nodes, which may undergo necrosis and become fluctuant. Although the invasion may stop there, Y pestis organisms often reach the bloodstream and become widely disseminated. Hemorrhagic and necrotic lesions may develop in all organs; meningitis, pneumonia, and serosanguineous pleuropericarditis are prominent features.
Primary pneumonic plague results from inhalation of infective droplets (usually from a coughing patient), and it is characterized by hemorrhagic consolidation, sepsis, and death.
The clinical manifestations of plague depend on the route of exposure. After an incubation period of 2–7 days, there is high fever and painful lymphadenopathy, commonly with greatly enlarged, tender nodes (buboes) in the neck, groin, or axillae. This is the bubonic form of the disease. Vomiting and diarrhea may develop with the early septicemic form of disease. Later, disseminated intravascular coagulation leads to hypotension, altered mental status, and renal and cardiac failure. Terminally, signs of pneumonia and meningitis can appear, and Y pestis multiplies intravascularly and can be seen in blood smears. Primary pneumonic plague results from direct inhalation of organism into the lung. Patients often have a fulminant course with chest pain, cough, hemoptysis, and severe respiratory distress.
Plague should be suspected in febrile patients who have been exposed to rodents in known endemic areas. Rapid recognition and laboratory confirmation of the disease are essential to institute lifesaving therapy.
Blood is taken for culture and aspirates of enlarged lymph nodes for smear and culture. Acute and convalescent sera may be examined for antibody levels. In pneumonia, sputum is cultured; in possible meningitis, cerebrospinal fluid is taken for smear and culture.