FIGURE 21-1. Hypothalamic-pituitary-ovarian axis (GnRH = gonadotropin-releasing hormone; LH = luteinizing hormone; FSH = follicle-stimulating hormone; (+) = stimulation of hormone secretion; (-) = inhibition of hormone secretion).
MENSTRUAL CYCLE
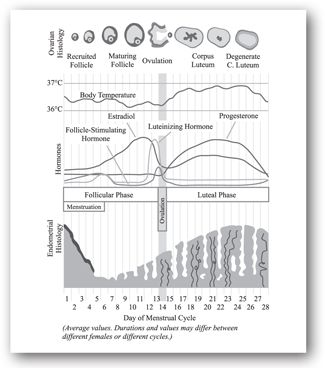
The reproductive cycle is divided into three phases: menstruation and the follicular phase, ovulation, and the luteal phase.1 These three phases referring to the status of the ovary during the reproductive cycle are depicted in Figure 21-1. The endometrium has the proliferative and secretory phases.
FIGURE 21-2. The menstrual cycle.
Phase I. Menstruation and the follicular phase. The first day of menstrual bleeding is considered day 1 of the typical 28 day menstrual cycle. During menstruation, the endometrium is sloughed in response to progesterone withdrawal. This is accompanied by the development of a new follicle during the follicular phase, with renewal of the endometrial lining of the uterus in preparation for implantation of an embryo. Women usually menstruate for 3–5 days.
Menstruation marks the beginning of the follicular phase of the cycle. With the beginning of menstruation, plasma concentrations of estradiol, progesterone, and LH reach their lowest point. In response to this reduction in negative feedback at the pituitary gland, FSH is increased at the beginning of menstruation. The increase in FSH begins approximately 2 days before the onset of menstruation. Under the influence of FSH, the granulosa cells begin to secrete estradiol.
Estradiol begins to rise in plasma by the fourth day of the cycle. Estradiol stimulates LH receptors on the theca cells, further increasing secretion of androgen precursors, which are converted by aromatase to estradiol in granulosa cells. The upregulation of LH receptors and hormone production prepares the granulosa and theca cells for progesterone synthesis after ovulation.
With rising estradiol levels, there is negative feedback to the pituitary gland to decrease the release of FSH and positive feedback to the pituitary gland to increase the release of LH. During the early follicular phase of the cycle, the FSH:LH ratio is <1; as the cycle progresses, the FSH:LH ratio becomes >1, demonstrating both positive and negative feedback effects of estradiol on the pituitary gland.
Phase II. Ovulation. As the dominant follicle secretes more and more estradiol, there is marked positive feedback to the pituitary gland to secrete LH. By days 11 to 13 of the normal cycle, an LH surge occurs, which triggers ovulation. Ovulation occurs within 30–36 hours of the LH surge, causing the oocyte to be expelled from the follicle and the follicle to be converted into corpus luteum to facilitate progesterone production during the remainder of the cycle. In addition, there is a slight increase in the basal body temperature (BBT) after ovulation.
Phase III. Luteal phase. The luteal phase of the menstrual cycle is characterized by a change in secretion of sex steroid hormones from estradiol predominance to progesterone predominance. As FSH rises early in the cycle, stimulating production of estradiol, additional LH receptors are created in the granulosa cells and then theca cells. With the LH surge at the time of ovulation, LH facilitates production of progesterone.
The production of progesterone begins approximately 24 hours before ovulation and rises rapidly thereafter. A maximum production of progesterone occurs 3–4 days after ovulation and is maintained for approximately 11 days following ovulation. If fertilization and implantation do not occur, progesterone production diminishes rapidly, initiating events leading to the beginning of a new cycle.
Adequate progesterone production is necessary to facilitate implantation of the fertilized oocyte into the endometrium and to sustain pregnancy into the early first trimester. If the initial rise in FSH is inadequate and the LH surge does not achieve maximal amplitude, an “inadequate luteal phase” can occur, resulting in progesterone production that is inadequate to facilitate implantation of a fertilized oocyte or to sustain pregnancy.
The corpus luteum has a fixed life span of 13–14 days unless pregnancy occurs. If the oocyte becomes fertilized and implants within the endometrium, the early pregnancy begins secreting human chorionic gonadotropin (hCG), which sustains the corpus luteum for another 6–7 weeks.
Physiologic plasma levels of progesterone exert negative feedback on pituitary secretion of both FSH and LH. During the luteal phase of the cycle, both FSH and LH are suppressed to low levels. As the corpus luteum fails and progesterone secretion diminishes, FSH begins to rise to prepare a woman for the next reproductive cycle.
AMENORRHEA
Amenorrhea is the absence or abnormal cessation of the menses.2 Primary and secondary amenorrhea describe the occurrence of amenorrhea before and after menarche, respectively. Primary amenorrhea can be diagnosed if a patient has normal secondary sexual characteristics but no menarche by 16 years of age.3 Secondary amenorrhea is the absence of menses for 3 months in women with previously normal menstruation and for 9 months in women with previous oligomenorrhea (scant menses). Secondary amenorrhea is more common than primary amenorrhea.4 The reader is referred to other texts for the evaluation of primary amenorrhea.
The prevalence of amenorrhea not due to pregnancy, lactation, or menopause is approximately 3% to 4%.2,4 History, physical examination, and measurement of FSH, thyroid-stimulating hormone (TSH), and prolactin will identify the most common causes of amenorrhea. Table 21-1 illustrates how symptoms elicited from a patient history assist in diagnosing the cause of amenorrhea.3,5
CNS = central nervous system; D&C = dilation and curettage; LHRH = luteinizing hormone-releasing hormone; PID = pelvic inflammatory disease; PCOS = polycystic ovary syndrome.
Source: Adapted from references 3 and 5.
During the physical examination, the clinician should note the presence of galactorrhea, thyromegaly, or other evidence of hypothyroidism or hyperthyroidism, hirsutism, acne, or signs of virilization.6 In addition, the patient’s body mass index (BMI) should be calculated. A BMI >20 may indicate hypothalamic ovulatory dysfunction, such as occurs with anorexia or other eating disorders. The presence of breast development suggests there has been previous estrogen activity. Excessive testosterone secretion is suggested most often by hirsutism and rarely by increased muscle mass or signs of virilization.
The combination of amenorrhea and galactorrhea strongly correlates with hyperprolactinemia. The history and physical examination should include a thorough assessment of the external and internal genitalia. Table 21-2 illustrates how physical examination findings assist in diagnosing the cause of amenorrhea.3,5
BMI = body mass index; PCOS = polycystic ovary syndrome.
Source: Adapted from references 3 and 5.
Hypothyroidism. Although other clinical signs of thyroid disease are usually noted before amenorrhea presents, abnormal thyroid hormone levels can affect prolactin levels. Treatment of hypothyroidism should restore menses, but this may take several months.7 Table 21-3 provides differential diagnoses of anovulatory disorders and associated serum laboratory findings.8
CAH = congenital adrenal hyperplasia; FSH = follicle-stimulating hormone; LH = luteinizing hormone; PCOS = polycystic ovary syndrome.
aNormal = ↔; mildly reduced = ↓; moderately reduced = ↓↓; significantly reduced = ↓↓↓; mildly elevated =↑; moderately elevated =↑↑; significantly elevated = ↑↑↑
Source: Adapted from reference 8.
Hyperprolactinemia. A patient with markedly elevated prolactin levels, galactorrhea, headaches, or visual disturbances should receive imaging tests to rule out a pituitary tumor. Adenomas are the most common cause of anterior pituitary dysfunction.9 A prolactin level more than 100 ng/mL suggests a prolactinoma, and a magnetic resonance imaging (MRI) should be performed. If tumor is excluded as the cause, medications (e.g., oral contraceptive pills, antipsychotics, antidepressants, antihypertensives, histamine (H2) blockers, and opiates) are the next most common cause of hyperprolactinemia. Medications usually increase prolactin levels to >100 ng/mL.9
In most amenorrheic women with hyperprolactinemia, prolactin levels do not decline without treatment, and the amenorrhea does not resolve as long as the prolactin levels remain elevated.9 In the absence of another organic condition, dopamine agonists (e.g., bromocriptine) are the preferred treatment of hyperprolactinemia with or without a pituitary tumor.2,10,11
Uterine outflow obstruction. The most common cause of outflow obstruction in secondary amenorrhea is Asherman syndrome (intrauterine scarring usually from curettage or infection).3 Certain gynecologic procedures can help diagnose Asherman syndrome. Other causes of outflow tract obstruction include cervical stenosis and obstructive fibroids or polyps.
Functional (hypothalamic) amenorrhea. Functional disorders of the hypothalamus or higher centers are the most common reason for chronic anovulation. Psychogenic stress, weight changes, undernutrition, and excessive exercise are frequently associated with functional hypothalamic amenorrhea, but the pathophysiologic mechanisms are unclear. More cases of amenorrhea are associated with weight loss than with anorexia, but amenorrhea with anorexia nervosa is more severe.11 (See Minicase 1.) Women involved in competitive sports activities have a threefold higher risk of primary or secondary amenorrhea than others, and the highest prevalence is among long-distance runners.14
Amenorrhea Secondary to Anorexia Nervosa
KIMBERLY M., A 20-YEAR-OLD WOMAN, comes into office for a urine pregnancy test due to no menses for about 2 months. Kimberly M. has no significant past medical history. Further questioning reveals she has lost a total of 30 pounds over the past 4 months and complains of recent acne. Her blood pressure is 100/63; her heart rate is 55 BPM; her temperature is 96.8° F; and her weight is 116 lb. Laboratory tests are as follows: urine hCG negative, FSH 1 million International Units/mL (5–25 million International Units/mL, follicular phase, LH 3 million International Units/mL (5–25 million International Units/mL, follicular phase), prolactin 20 ng/mL (1–25 ng/mL), testosterone 20 ng/dL (20-60 ng/dL).
Question: What is the most likely diagnosis?
Discussion: Kimberly M. complains of amenorrhea. Pregnancy is not a cause of amenorrhea because her urine hCG is negative. She has hypotension, bradycardia, and hypothermia—all indicative of anorexia. Also, she reports significant weight loss in a relatively short period of time.
Treatment of hypothalamic amenorrhea depends on the etiology. Women with excessive weight loss should be screened for eating disorders and treated if anorexia nervosa or bulimia nervosa is diagnosed. Menses usually will return after a healthy body weight is achieved.15 With young athletes, menses may return after a modest increase in caloric intake or a decrease in athletic training. Women with hypothalamic amenorrhea are also susceptible to the development of osteoporosis.16 Unless the primary cause can be easily treated, cyclic estrogen-progestin therapy or oral contraceptive pills should be initiated to prevent excessive bone loss.
Ovarian failure. Approximately 1% to 5% of women have premature ovarian failure, a condition where persistent estrogen deficiency and elevated FSH levels occur prior to the age of 40 years, resulting in amenorrhea.17 Ovarian failure is confirmed by documenting an FSH level persistently in the menopausal range.2 Iatrogenic causes of premature ovarian failure, such as chemotherapy and radiation therapy for malignancy, have a potential for recovery. Ovarian function may fluctuate, with an increasingly irregular menstrual cycle before permanent ovarian failure. The resulting fluctuations in gonadotropin levels account for the lack of accuracy associated with a single FSH value.18 Women with ovarian failure should be offered estrogen and progestin treatment to promote and maintain secondary sexual characteristics and reduce the risk of developing osteoporosis.
Menopause represents a type of “physiologic” ovarian failure, which is defined as the cessation of menses for at least 12 months. The climacteric or perimenopause are the periods of waning ovarian function before menopause (i.e., the transition from the reproductive to the nonreproductive years).1 The average age for menopause in the United States is between 50 and 52 years of age (median 51.5), with 95% of women experiencing this event between the ages of 44 and 55.1 (See Minicase 2.)
Premature Menopause
BECCA T., A 38-YEAR-OLD WOMAN, returns for further testing due to complaints of irregular menses over the past 8 months, loss of sexual desire, vaginal dryness, and episodes of warmth and sweating throughout the day. Her past medical history includes breast cancer, for which she underwent chemotherapy and radiation. On examination, her blood pressure is 120/68, her heart rate is 90 BPM, and her temperature is 100°F. The thyroid gland is normal to palpation. Cardiac and lung examinations are unremarkable. Breast examination reveals symmetrical breasts, without masses or discharge. Examination of the external genitalia does not reveal any masses. Her laboratory values were obtained on day 3 of menses and are as follows: FSH 23 million International Units/mL (5–25 million International Units/mL, follicular phase), LH 96 million International Units/mL (5–25 million International Units/mL, follicular phase).
Question: What is the most likely diagnosis?
Discussion: Becca T. complains of irregular menses, vaginal dryness, and intermittent sensations of warmth and sweating. This constellation of symptoms is consistent with the premature menopause. Elevated FSH and LH levels confirm the diagnosis. Due to this woman’s age and medical history, premature menopause is likely. However, even when gonadotropins are in the menopausal range, as in this case, ovulation can still be occurring, albeit irregularly and unpredictably. Thus, it is best to draw FSH and LH levels during the follicular phase because they reach their lowest point during this phase. Hot flushes, which are typical vasomotor changes due to decreasing estrogen levels, are associated with skin temperature elevation and sweating lasting for 2–4 minutes. The low estrogen concentration also has an effect on the vagina by decreasing the epithelial thickness, leading to atrophy and dryness. Although this woman’s estradiol level would mostly likely be low, it is not a reliable indicator of menopausal transition because estradiol levels are prone to cyclical fluctuations, as shown in Figure 21-2. Thus, it is not necessary to draw an estradiol level.
Treatment for hot flashes includes hormone therapy with estrogen. Certain antidepressants, such as venlafaxine, can help with vasomotor symptoms as well.53 When the woman still has her uterus, the addition of progestin to estrogen replacement is important for preventing endometrial cancer. Because of significant increased risks of breast cancer, heart disease, pulmonary embolism, and stroke are associated with hormone therapy, estrogens are not the best treatment for vasomotor symptoms for Becca T.20,21
During perimenopause, the ovarian follicles diminish in number and become less sensitive to FSH.1 The process of ovulation becomes increasingly inefficient, less regular, and less predictable than in earlier years. Initially, a woman may notice a shortening of the cycle length. With increasing inefficiency of the reproductive cycle, the follicular phase shortens, but the luteal phase is maintained at normal length. With the passing of time, some cycles become anovulatory. As menopause approaches, the remaining follicles become almost totally resistant to FSH. The process of ovulation ceases entirely, and cyclic hormone production ends with menopause.
Serum FSH levels begin to rise with diminishing ovarian function; the elevation is first detected in the follicular phase. This early follicular phase rise in FSH has been developed into an endocrine test of ovarian functional reserve in which one draws an FSH level on cycle day 3. With a further decrease in ovarian function, the FSH level will be elevated consistently throughout the menstrual cycle in perimenopausal women and in women after oophorectomy. Table 21-4 depicts serum hormones according to age.19
FSH = follicle-stimulating hormone; LH = luteinizing hormone.
Source: Adapted from reference 19.
The postmenopausal ovary is not quiescent. Under the stimulation of LH, androgens (i.e., testosterone and androstenedione) are secreted. Testosterone concentrations decline after menopause but remain 2 times higher in menopausal women with intact ovaries than in those with ovaries removed (oophorectomy). Estrone is the predominant endogenous estrogen in postmenopausal women and is termed extragonadal estrogen because the concentration is directly related to body weight and androstenedione is converted to estrone in adipose tissue. Table 21-5 compares concentrations of androgens and estrogens in premenopausal women, postmenopausal women, and women after oophorectomy.1
Source: Adapted from reference 8.
Polycystic ovary syndrome. PCOS affects approximately 6% of women of reproductive age and is the most frequent cause of anovulatory infertility.22,23 Clinical signs include those associated with a hyperandrogenic anovulatory state, including hirsutism and acne. Approximately 70% of affected women manifest growth of coarse hair in androgen-dependent body regions (e.g., sideburn area, chin, upper lip, periareolar area, chest, lower abdominal midline and thigh) as well as upper-body obesity with a waist-to-hip ratio of >0.85.8 Patients usually retain normal secondary sexual characteristics and rarely exhibit virilizing signs such as clitoromegaly, deepening of the voice, temporal balding or masculinization of body habitus. Suggested diagnostic criteria for PCOS are provided in Table 21-6.8 (See Minicase 3.)
CAH = congenital adrenal hyperplasia; FSH = follicle-stimulating hormone; LH = luteinizing hormone; PCOS = polycystic ovary syndrome.
Source: Adapted from reference 8.
Polycystic Ovary Syndrome
SARAH T., A 23-YEAR-OLD UNMARRIED WOMAN, presents with a complaint of an increased need to wax her upper lip and chin. Her history includes several irregular menstrual cycles with occasional amenorrhea. She is on hydrochlorothiazide for hypertension, is not sexually active, and otherwise feels well. She is concerned about the irregularity of her menses and future fertility. Pelvic ultrasound reveals a solid appearing right ovary 5–6 cm in diameter. Laboratory findings on day 5 of her menstrual cycle are as follows: urine hCG negative, FSH 15 million International Units/mL (5–25 milliunits/mL, follicular phase), LH 50 million International Units/mL (5–25 milliunits/mL, follicular phase), prolactin 35 ng/mL (1–25 ng/mL), total testosterone 75 ng/dL (20–60 ng/dL).
Question: What is the most likely cause of her symptoms?
Discussion: Polycystic ovary syndrome is the most common cause of Sarah T.’s complaints. The increased hair growth revealed androgen excess in general are symptoms consistent with those of PCOS. Testosterone levels are elevated, LH and prolactin are slightly increased, and the LH to FSH ratio is 3:1, all suggestive of PCOS. Urine hCG ruled out pregnancy. Prolactinoma was not a causative factor because the prolactin level was only mildly elevated. It was not necessary to obtain dehydroepiandrosterone sulfate (DHEAS) levels to determine the presence of a virilizing adrenal tumor because the testosterone level was <200 ng/dL. In order to check for glucose intolerance and the prevention of cardiovascular disease, a glucose level, glucose tolerance test, and lipid profile should be obtained in Sarah T.
Women with PCOS often develop polycystic ovaries as a function of a prolonged anovulatory state. Follicular cysts are observed on ultrasound in more than 90% of women with PCOS, but they are also present in up to 25% of normal women.23–26 Although PCOS is primarily a clinical diagnosis, some debate exists about whether the diagnosis should be based on assays of circulating androgens rather than clinical signs and symptoms of hirsutism and/or acne, as well as glucose abnormality. This is because a substantial number of women with PCOS have no overt clinical signs of androgen excess.8
Gonadotropin abnormalities in PCOS include elevated levels of testosterone and LH or an elevated LH-to-FSH ratio, an increased LH pulse frequency and altered diurnal rhythm of LH secretion. The LH-to-FSH ratio is used to facilitate diagnosis, and many researchers consider an LH-to-FSH ratio of 3:1 or greater diagnostic of the syndrome.28 Although serum testosterone levels are mildly-to-moderately elevated in women with PCOS, testosterone levels are also measured to rule out virilizing tumors. Prolactin levels are usually measured to exclude a possible prolactinoma. Suggested laboratory and radiologic evaluation for PCOS is provided in Table 21-7.8
17-OHP = 17 alpha-hydroxyprogesterone; DHEAS = dehydroepiandrosterone sulfate; FSH = follicle-stimulating hormone;
hCG = urine human chorionic gonadotropin; LH = luteinizing hormone;
PCOS = polycystic ovary syndrome.
aSuggested only in selected patients.
Source: Adapted from reference 8.
Women with PCOS should also be screened for abnormal glucose metabolism because of an association with glucose intolerance. To aid in the possible prevention of cardiovascular disease, lipid abnormalities and blood pressure should be monitored annually.
The primary treatment for PCOS is weight loss through diet and exercise. Modest weight loss can lower androgen levels, improve hirsutism, normalize menses, and decrease insulin resistance.28 Use of oral contraceptive pills or cyclic progestational agents can help maintain a normal endometrium. The optimal cyclic progestin regimen to prevent endometrial cancer is unknown, but a monthly 10- to 14-day regimen is recommended.28 Insulin sensitizing agents such as metformin can reduce insulin resistance and improve ovulatory function.28,29
Hyperandrogenism. Significantly elevated testosterone or DHEAS levels may also indicate an androgen-secreting tumor (ovarian or adrenal). Levels of 17 alpha-hydroxyprogesterone (17-OHP) can help diagnose adult-onset congenital adrenal hyperplasia (CAH).28 Cushing syndrome is rare; therefore, patients should only be screened when characteristic signs and symptoms (e.g., striae, buffalo hump, significant central obesity, easy bruising, hypertension, proximal muscle weakness) are present.28
Estrogen status. If TSH and prolactin levels are normal, a progesterone challenge test can help detect endogenous estrogen that is affecting the endometrium. A withdrawal bleed usually occurs 2–7 days after the challenge test.3 A negative progesterone challenge test signifies inadequate estrogenization, and requires further followup for other underlying causes.
HIRSUTISM AND VIRILIZATION
Hirsutism is defined as the presence of excess terminal hair in androgen-dependent areas of a woman’s body and occurs in up to 8% of women.30–32 The disorder is a sign of increased androgen action on hair follicles from increased circulating levels of androgens (endogenous or exogenous) or increased sensitivity of hair follicles to normal levels of circulating androgens.
Infrequently, hirsutism may signal more serious pathology, and clinical evaluation should differentiate benign causes from tumors or other conditions that require specific treatment. Hair growth varies widely among women and distinguishing normal variations of hair growth from true hirsutism is important.
While 60% to 80% of women with hirsutism have increased levels of circulating androgens, degrees of hirsutism correlate poorly with androgen levels.33 The ovary is the major source of increased levels of testosterone in women who have hirsutism.30 Dehydroepiandrosterone sulfate is an androgen that arises almost exclusively from the adrenal gland but is an uncommon cause of hirsutism. Nearly all circulating testosterone is bound to sex hormone binding globulin (SHBG) and albumin, with free testosterone being the most biologically active form. When elevated insulin levels are present, SHBG levels decrease while free testosterone levels increase. (See Minicase 4.)
Hirsutism Secondary to Adnexal (Ovarian) Tumor
KELLY S., A 45-YEAR-OLD PAROUS WOMAN, has noticed increasing hair growth on her face and abdomen over the past 6 months. She denies using steroid medications, weight change, or a family history of hirsutism. Her menses has been monthly with the exception of the past 3 months. Her past medical and surgical histories are unremarkable. On examination, her thyroid is normal to palpation. She has excess facial hair and male pattern hair on her abdomen. Acne is noted on the face. She also notes increased sweating and some thinning of her hair. Cardiac and pulmonary examinations are normal. Abdominal examination reveals no masses or tenderness. Examination of the external genitalia reveals possible clitoromegaly. Pelvic examination shows a normal uterus and cervix and an 8 cm, right adnexal mass. Her laboratory values obtained on the 4th day of her menstrual cycle are as follows: LH 10 (5–25 million International Units/mL, follicular phase), FSH 7(5–25 million International Units/mL, follicular phase), total testosterone 85 ng/dL (20–60 ng/dL), prolactin 13 ng/mL (1–25 ng/mL).
Question: What is the mostly likely diagnosis?
Discussion: Kelly S. has onset of excess male-pattern hair over the past 6 months, as well as features of virilism (clitoromegaly). This is evidence of excess androgens. The rapid onset suggests a tumor. Adrenal or ovarian tumors are possibilities. She has a large adnexal mass, so the diagnosis is straightforward. She has irregular menses because of an inhibition of ovulation by androgens. Kelly S. does not have the presentation of Cushing syndrome, such as hypertension, buffalo hump, abdominal striae, and central obesity. Likewise, she does not take any medications containing anabolic steroids (e.g., oxymetholone). Although PCOS is probably the most common cause of hyperandrogenism, it does not fit her clinical presentation because it usually presents with a gradual onset of hirsutism and irregular menses since menarche. Also, she does not have an LH:FSH ratio <3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree