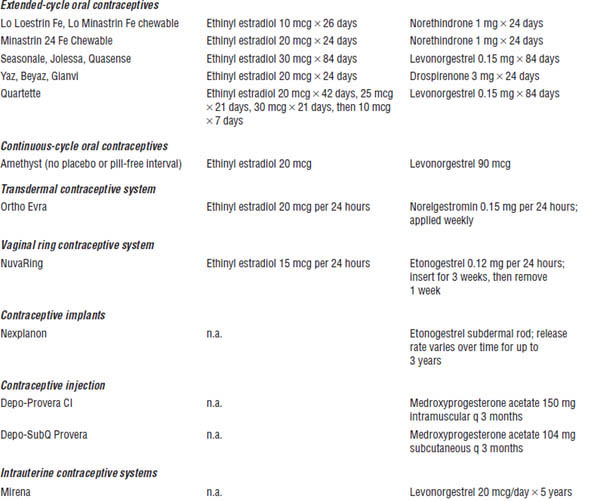
Table 19-1. Selected Hormone Therapy Products
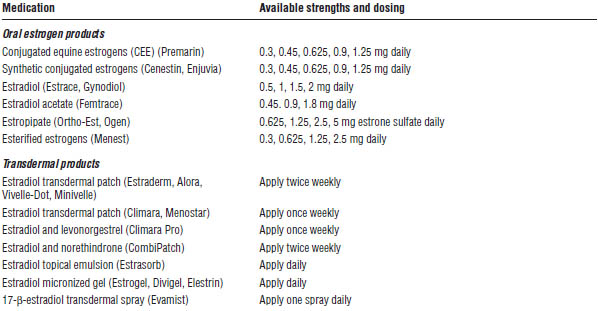
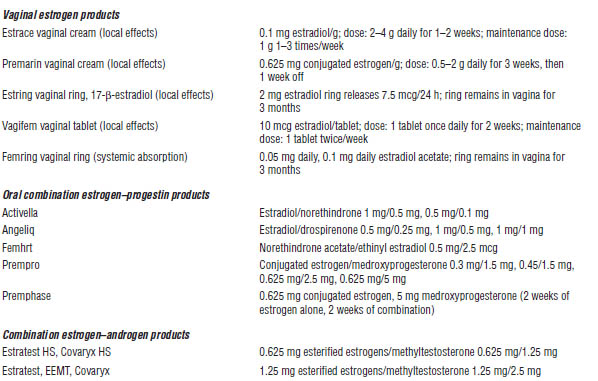
Adapted from Kalantaridou et al., 2014; Hester, 2014.
Combined estrogen–progestin therapy (EPT) includes progestin to prevent endometrial hyperplasia and cancer.
Patient instructions and counseling
■ Side effects of estrogen may be diminished by starting with a low dose and may be alleviated by changing products. Most side effects improve with time.
■ Side effects of progestin may be alleviated or diminished by changing products or changing from a continuous to a cyclic regimen.
■ Patients should be instructed to immediately report any unusual vaginal bleeding.
■ Patients should be instructed to contact their physician promptly if any of the following events occur:
• Abdominal tenderness, pain, or swelling
• Disturbances of vision or speech
• Dizziness or fainting
• Lumps in the breast
• Numbness or weakness in an arm or leg
• Severe vomiting or headache
• Sharp chest pain or shortness of breath
• Sharp pain or swelling in the calves
Adverse drug events
Increased risks for venous thromboembolism, stroke, coronary heart disease, and breast cancer were identified in the Women’s Health Initiative (WHI) trial with postmenopausal women receiving HT. The beneficial effects may include reductions in fractures and colorectal cancer. Both ET and EPT should be used at the lowest doses and for the shortest possible time period for women who are experiencing moderate to severe vasomotor symptoms or vulvovaginal atrophy. Consider topical estrogen, particularly if the primary complaint is vulvovaginal atrophy.
The following adverse effects are most common with estrogen:
■ Breast tenderness
■ Heavy or irregular bleeding
■ Headache
■ Nausea
The following adverse effects are most common with progestin:
■ Depression
■ Headache
■ Irritability
Drug–drug and drug–disease interactions
Estrogen may exacerbate illness in the following disease states:
■ Depression
■ Hypertriglyceridemia (avoid by using transdermal product)
■ Thyroid disorder (patients may require an increased dose of thyroid supplement)
■ Impaired hepatic function (poor metabolism of estrogens)
■ Cardiovascular disorders (coronary heart disease and venous thromboembolism risk may be increased with estrogens)
■ Cholelithiasis
■ Gastroesophageal reflux disease
Interaction may result in decreased pharmacologic effect of estrogens:
■ Cytochrome P450 (CYP450) 3A4 inducers:
• Barbiturates
• Carbamazepine
• Rifampin
• St. John’s wort
• Phenytoin
Interaction may result in increased pharmacologic effect of estrogens:
■ CYP450 3A4 inhibitors:
• Azole antifungals
• Macrolide antibiotics
• Ritonavir
Parameters to monitor
Laboratory monitoring is not recommended. Patients should be monitored for symptom improvement, adverse effects, and appropriate health maintenance (e.g., annual mammograms).
Androgens (testosterone)
Mechanism of action
Androgens are the precursor hormones to estrogen production by the ovaries and peripheral sites. Ovarian testosterone production declines with menopause.
Androgens act at androgen receptor sites or exhibit action following conversion to estrogen. Androgen replacement improves deficiency-related symptoms (i.e., decreased sexual desire, decreased energy, diminished well-being).
Patient instructions and counseling
■ Testosterone therapy should be administered only to postmenopausal women who are receiving concurrent estrogen therapy.
■ The following are relative contraindications to testosterone therapy:
• Androgenic alopecia
• Hirsutism
• Moderate to severe acne
Adverse drug events
■ Fluid retention
■ Decreased high-density lipoprotein and triglycerides
■ Hepatic dysfunction
■ Hepatocellular carcinoma (prolonged use of high doses)
Parameters to monitor
■ Laboratory monitoring is not recommended.
Nondrug Therapy
Phytoestrogens
■ Phytoestrogens are plant compounds (isoflavones, lignans, coumestans).
■ Food sources of phytoestrogens include soy (milk, edamame, tofu); flaxseed; and alfalfa sprouts.
■ Phytoestrogens may improve vaginal symptoms, lipids, weight, and blood pressure and may slow loss of bone mineral density.
■ No evidence supports improvement in other symptoms of menopause (i.e., hot flashes, depression, anxiety, headache, myalgia).
19-4. Contraception
Contraception is the prevention of pregnancy by one of two methods:
■ Preventing implantation of the fertilized ovum in the endometrium
■ Inhibiting contact of sperm with mature ovum
Prescription Contraceptive Options
Combined hormonal contraception (CHC)
■ Combined oral contraceptive (COC)
• COC contains estrogen plus progestin.
• It may be monophasic, biphasic, triphasic, or four-phasic.
• Monophasic may be used in extended regimens.
■ Transdermal contraceptive patch
• Apply to skin once weekly.
• Patch may be used for extended regimens.
■ Contraceptive vaginal ring
• Insert once monthly, leave in for 3 weeks, and then remove for 1 week.
• Ring may be used for extended regimens.
Progestin-only hormonal contraception
■ Progestin-only oral contraceptive (minipill)
• Appropriate for breast-feeding women
• Less efficacious than combined oral contraceptives
• Free of cardiovascular risks associated with estrogen-containing products, but may cause weight gain
■ Injectable depot medroxyprogesterone acetate (DMPA)
• Intramuscular or subcutaneous
• Injection every 3 months
• Weight gain, menstrual irregularity, and osteoporosis
■ Intrauterine device (IUD)
• May contain copper (Paragard) or levonorgestrel (Mirena, Skyla)
• Contraindicated in women who have pelvic inflammatory disease, are immunocompromised, or have a history of ectopic pregnancy
■ Subdermal progestin-releasing implant
• Etonogestrel (Nexplanon)
• Effective up to 3 years
• Possible decrease in efficacy in women over 130% of ideal body weight
• May cause menstrual irregularity
Estrogens and Progestins Used in Prescription Contraceptives (Table 19-2)
■ Estrogens:
• Ethinyl estradiol, estradiol valerate
• Mestranol
■ Progestins:
• Desogestrel
• Norgestrel, levonorgestrel
• Ethynodiol diacetate
• Norethindrone, norethindrone acetate, norethynodrel
• Dienogest
Table 19-2. Prescription Contraceptive Products
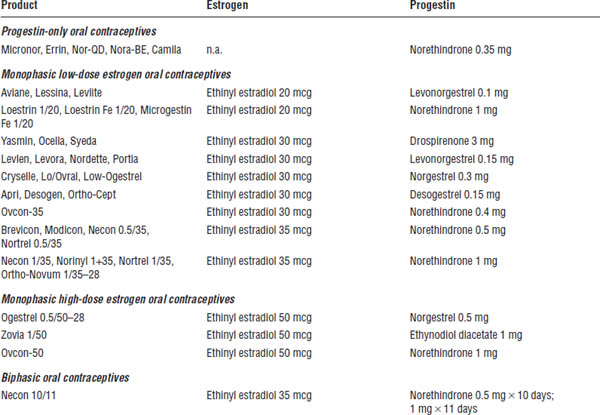
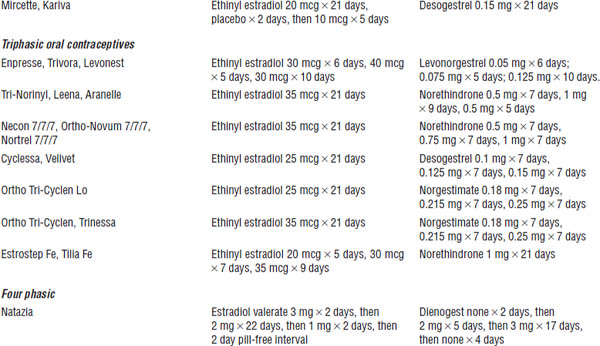
Adapted from Shrader, Ragucci, 2014; O’Mara, 2013.
n.a., not applicable.
Drospirenone
■ Progestin with progestogenic, antiandrogenic, and antimineralocorticoid activity
Drug Therapy
Mechanism of action
Estrogens prevent development of a dominant follicle by suppression of follicle-stimulating hormone. They do not block ovulation.
Progestin inhibits ovulation. It contributes to the production of thick and impermeable cervical mucus. It also contributes to involution and atrophy of the endometrium.
Patient instructions and counseling
■ Efficacy is high (99 %) with perfect use, but it depends on adherence. (Efficacy is 92% with typical use.)
■ Oral contraceptives do not prevent the transmission of sexually transmitted diseases.
■ Patients should be educated on warning signs of serious complications:
• Severe abdominal pain
• Severe chest pain, shortness of breath, coughing up of blood
• Severe headache
• Eye problems (i.e., blurred vision, flashing lights, blindness)
• Severe leg pain in the calf or thigh
■ Patients should be advised to expect changes in characteristics of the menstrual cycle.
■ Use of a backup contraceptive method is advised if more than one dose is missed per cycle.
Adverse drug events
The World Health Organization (WHO) suggests refraining from prescribing COCs to women with the following diagnoses:
■ Breast cancer
■ Deep-vein thrombosis or pulmonary embolism
■ Cerebrovascular disease or coronary artery disease
■ Diabetes with nephropathy, neuropathy, retinopathy, or other vascular disease
■ Migraine headaches
■ Uncontrolled hypertension (≥ 160/90 mm Hg)
■ Breast-feeding women (< 6 weeks postpartum)
■ Liver disease
■ Pregnancy
■ Surgery with prolonged immobilization or any surgery on the legs
■ Age > 35 years and currently smoking (≥ 15 cigarettes a day)
■ Hypercoagulable states (e.g., factor V Leiden, protein C or S deficiency)
■ Complicated valvular heart disease (pulmonary hypertension, risk of atrial fibrillation, history of endocarditis)
■ Peripartum cardiomyopathy
■ Migraine, with aura
For these medical conditions, use of progestin-only oral contraceptives, DMPA, or an IUD may be an appropriate contraceptive choice.
The most common adverse drug events with COCs are as follows:
■ Nausea and vomiting (usually resolves within 3 months)
■ Breakthrough bleeding, spotting, amenorrhea, altered menstrual flow
■ Melasma (hyperpigmentation of the skin, usually on the face)
■ Headache or migraine
■ Weight change or edema
Serious but less common effects are venous thrombosis, pulmonary embolism, myocardial infarction, coronary thrombosis, arterial thromboembolism, and cerebral thrombosis.
Potential hormonal effects are associated with an imbalance in estrogen and progestin (Table 19-3).
Drug–drug and drug–disease interactions
Interaction with the following drugs may result in decreased pharmacologic effect of oral contraceptives:
■ Ampicillin, griseofulvin, sulfonamides, and tetracycline
■ Anticonvulsants (barbiturates, carbamazepine, felbamate, phenytoin, topiramate)
■ Some antiretroviral medications (e.g., nevirapine, lopinavir + ritonavir)
■ Rifampin
Table 19-3. Potential Hormonal Effects Associated with an Imbalance in Estrogen or Progestin
Type of imbalance | Effect |
| |
Too much estrogen | ■ Breast tenderness, fullness ■ Nausea ■ Edema and bloating ■ Hypertension ■ Melasma ■ Headache |
| |
Too much progestin | ■ Acne or oily scalp ■ Breast tenderness ■ Depression or irritability ■ Hypomenorrhea ■ Increased appetite and weight gain ■ Fatigue ■ Constipation |
| |
Too little estrogen | ■ Breakthrough bleeding (early, days 1–9 of cycle) ■ Hypomenorrhea ■ Vasomotor symptoms |
| |
Too little progestin | ■ Amenorrhea ■ Breakthrough bleeding (late, days 14–21 of cycle) ■ Hypermenorrhea |
Adapted from Shrader, Ragucci, 2014; El-Ibiary, White, 2010.
Interaction with these drugs may result in increased plasma levels of oral contraceptives:
■ Atorvastatin
■ Vitamin C
■ CYP450 3A4 inhibitors
Interaction with the following drugs may result in decreased pharmacologic effect of the interacting drug:
■ Anticoagulants (estrogens have a procoagulant effect)
■ Some benzodiazepines (e.g., lorazepam, oxazepam, temazepam)
■ Methyldopa
■ Phenytoin
Interaction with the following may result in increased pharmacologic effect of interacting drug:
■ Tricyclic antidepressants
■ Some benzodiazepines (other than the benzodiazepines previously listed)
■ β-blockers
■ Theophylline
Parameters to monitor
Patients must monitor themselves for warning signs of serious complications previously listed. Laboratory monitoring is not recommended with use of oral contraceptives.
Nondrug Therapy
Nondrug therapy includes use of the following:
■ Condoms
■ Diaphragms
■ IUDs
■ Spermicides
19-5. Osteoporosis
Osteoporosis is characterized by low bone mineral density and deterioration of bone tissue, increasing fragility of bone, and subsequent risk of fracture.
Diagnostic Criteria
Dual-energy x-ray absorptiometry scans are used to diagnose osteoporosis. T-scores are used to guide diagnosis and decision to treat osteoporosis. The WHO classification of bone mass is based on T-scores:
■ Osteopenia: T-score −1 to −2.5 standard deviations below the young adult mean
■ Osteoporosis: T-score below −2.5 standard deviations below the young adult mean
Risk factors for osteoporosis are as follows:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree