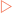chapter 2 Water: the solvent of life
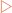 The concentration of H+ ions in a solution can be expressed as a pH value, with below 7 meaning the solution is acidic and above 7 meaning it is basic.
The concentration of H+ ions in a solution can be expressed as a pH value, with below 7 meaning the solution is acidic and above 7 meaning it is basic.Water is an amazing substance. Most known organisms are predominantly made from, and live in an environment dominated by, water. Approximately 75% of the surface of the Earth is covered by water, and although most of it is liquid it can exist as solid (ice) and vapour as well. This makes water the only common substance to naturally occur in the three main states of matter: solid, liquid and gas. Fossil evidence suggests that life began in water, and it is probably for this reason that cells and their environment mainly contain water. The properties of water have helped to mould the evolution of life on Earth, in form, function and chemistry.
Why is water special?
Generally we take water for granted. For most of us it is readily available and we tend to think about it only when it is in short supply. Water is a small and apparently simple molecule, made from two atoms of hydrogen and one atom of oxygen. Each hydrogen atom is attached to the oxygen atom by a single covalent bond to form a roughly V-shaped molecule. However, because the oxygen atom is more electronegative than the hydrogen atoms, the distribution of electrons in the bonds is uneven, with a higher density close to the oxygen. This means that the oxygen atom is slightly negative, and the hydrogen atoms are slightly positive (Fig 2-1), making water a polar molecule. This polarity allows water molecules to engage in extensive hydrogen bonding and gives water unique properties for such a small molecule.
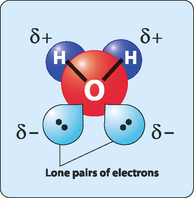
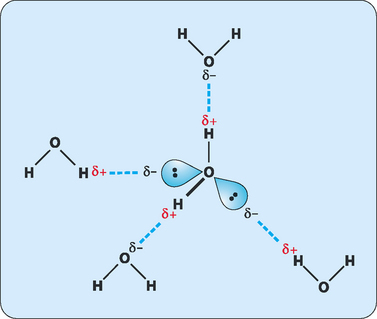
If the physical and chemical properties of water are compared with similar small molecules it is obvious that water acts very differently to the other small molecules (Table 2-1). A comparison of the ability of these molecules to undergo hydrogen bonding helps us to rationalise these properties. The covalent bonds in methane are nonpolar so there is no possibility of hydrogen bonding. Ammonia and hydrogen fluoride have the ability to make two hydrogen bonds and hydrogen bonding in hydrogen sulfide is very weak. This makes all of these molecules much more volatile than water, which has the ability to make four hydrogen bonds: two through the slightly positive hydrogen atoms and two through the lone pairs of electrons on the oxygen atom (Fig 2-2). However, these hydrogen bonds are fragile in the liquid state, forming and breaking frequently (about 109 times per second). This means that at any given time a large number of water molecules are engaged in hydrogen bonds with nearby molecules. These extensively hydrogen-bonded networks give water its anomalous properties.
Water and life processes
In Table 2-1 it was demonstrated that the polarity of water gives it much higher melting and boiling points than similar molecules. This polarity also makes water an extremely versatile solvent. If common salt (NaCl) is added to water and mixed, the salt dissolves to form a solution, a homogenous mixture of two substances that is visually uniform throughout. This occurs because the sodium and chloride ions which make up each salt crystal become exposed to the water molecules. The slightly negatively charged oxygen atoms become attracted to the positively charged sodium ions (cations) (Fig 2-3). Similarly the slightly positive hydrogen atoms are attracted to the negatively charged chloride ions (anions). The water molecules eventually completely surround each ion and form a hydration shell, which isolates the ions from each other. Eventually all the sodium and chloride ions become dissolved in this manner. In this salt solution, the water acts as the solvent (the substance present in a larger amount) and the salt as the solute (the substance present in a smaller amount).
Concentration = mass of solute (g)/volume of solvent (L)
However, this sort of concentration is not particularly useful for scientists in some situations and a different form of concentration is used. To do this the molar mass of the solute must first be calculated. For example, if common salt (NaCl) is the solute then its molar mass is 58.5 daltons (mass for sodium is 23 and chlorine is 35.5 as it takes into account the relative abundance of the isotopes). To make this system practicable scientists measure compounds in moles (mol), rather than exact numbers of atoms, elements, molecules or compounds. In fact the exact number is 6.02 × 1023, referred to as Avogadro’s number
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree