Fig. 30.1.
Lichen simplex chronicus . Acanthosis, hyperkeratosis, and chronic inflammation in the dermis are typical features.
♦
Hyperkeratosis, parakeratosis, and hypergranulosis may be present
♦
Surface erosion with exocytosis may be seen secondary to scratching
♦
If there are no inflammation and dermal collagen zone, the diagnosis of squamous cell hyperplasia is pertinent (see “Squamous Cell Hyperplasia”)
Lichen Planus
♦
Lichen planus is an uncommon inflammatory disease of unknown etiology
♦
It typically involves vulvar vestibule and vagina. The affected areas may be markedly erythematous. Serosanguinous discharge may be present
♦
Involvement of vulva, oral mucosa, and vaginal mucosa is referred to as the vulvovaginal–gingival syndrome
♦
Diagnostic features of vulvar lichen planus include a lichenoid interface chronic inflammatory infiltrate (predominantly lymphocytic with no or rare plasma cells) and pointed rete ridges (Fig. 30.2A). Wedge-shaped hypergranulosis and hyperkeratosis may be prominent. Severe cases may have colloid bodies (civatte bodies) with keratinocyte liquification necrosis, bullae, and ulceration (Fig. 30.2B)
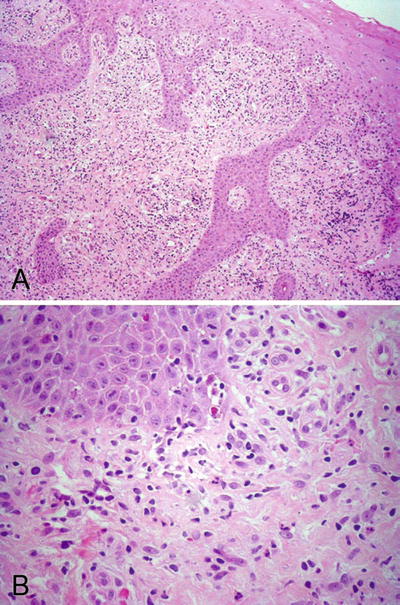

Fig. 30.2.
Lichen planus . (A) Lichenoid interface inflammation with band-like lymphoid infiltrate and acanthosis are seen. (B) Necrotic keratinocytes and colloid bodies are identified.
♦
Special stains for bacteria or fungi are negative
♦
Lichen planus can be difficult to differentiate from early lichen sclerosis (see below). Features favoring lichen planus include civatte bodies, wedge-shaped hypergranulosis, pinpoint rete ridges, and frequent mucosal involvement
Behcet Disease
Clinical
♦
Behcet disease occurs most commonly in Japan and Eastern Mediterranean countries. The mean age at onset is in the 30s. There is a slight male predominance
♦
Behcet disease is a clinical syndrome characterized by systemic vasculopathy and presents as a triad of oral aphthous ulcers, genital ulcers, or skin lesions such as pustules or erythema nodosa-like lesions and eye lesions such as uveitis or retinal vasculitis
♦
The vulvar and oral ulcers are often simultaneous; they characteristically heal and relapse
♦
Administration of appropriate, systemic colchicine and pentoxyfylline are the main therapies for vulvar and oral ulcers
Microscopic
♦
The cardinal pathologic finding is necrotizing vasculitis that may involve all calibers and types of vessels in the dermal and subcutaneous areas. The vasculitis may be associated with mural necrosis and thrombosis
Fox–Fordyce Disease
Clinical
♦
Fox–Fordyce disease is a disorder of the apocrine glands, commonly seen in the genital region and axilla. It usually occurs after puberty and presents as multiple pruritic and papular eruptions. About 90% of cases occur in women
♦
It is a chronic condition from inflammatory responses to the leakage of the apocrine secretion into the dermis. The condition may progress during pregnancy or after menopause
♦
No effective therapy is known
Microscopic
♦
A typical papule contains a hair follicle and apocrine sweat gland duct plugged by keratin in the center (Fig. 30.3)
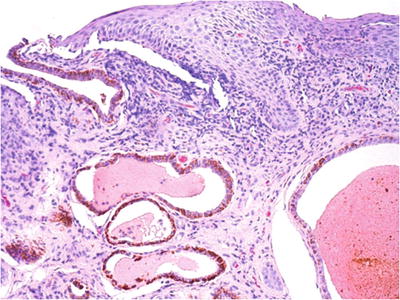

Fig. 30.3.
Fox–Fordyce disease . Apocrine gland dilatation associated with epidermal and dermal inflammation, acanthosis, and melanosis is demonstrated in this vulvar biopsy from a 17-year-old female.
♦
Intraepidermal vesicle formation may be seen, secondary to rupture of the intraepithelial portion of the duct and spongiosis
♦
Chronic inflammatory changes are present in the dermis
♦
Additional changes include dilation of apocrine gland acini and epidermal acanthosis
Hidradenitis Suppurativa
Clinical
♦
Hidradenitis suppurativa is a chronic debilitating disease of the apocrine glands, commonly occurs in the vulva, perineum, and axilla. It presents as deep-seated, painful, subcutaneous nodules. The lesions may progress toward surface that causes ulceration of the epidermis, draining sinus, and extensive scarring
♦
Intercourse may be extremely uncomfortable and even impossible due to the inflammatory reaction in the affected area
Microscopic
♦
In early stage, there is perifolliculitis with acute and chronic inflammatory infiltrate within the dermis (Fig. 30.4). Destruction of the epithelial appendages with sinus tract formation occurs at the later stages
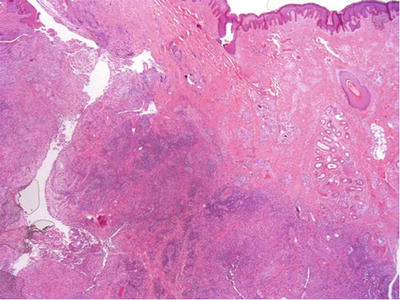

Fig. 30.4.
Hidradenitis suppurativa .
Infectious Lesions
Human Papilloma Virus Infections
Clinical
♦
Human papilloma virus (HPV) infection of the vulva is common and causes lesions like vulvar condyloma acuminata (venereal wart). Most vulvar condyloma acuminata are caused by HPV6 and some by HPV11. They are commonly associated with condylomas in the vagina and cervix, sometimes with condylomas of the perineal and perianal skin and the anal and urethral mucosa
♦
Grossly, condylomas vary from only colposcopically visible lesions to a large sessile or exophytic growth. They are often multiple and can become confluent. Typically, they involve labia majora and vestibules
♦
Patients’ hormonal and immune status can influence size of the lesions. Vulvar condylomas may enlarge or increase in number during pregnancy and regress postpartum. However, there is no particular association between oral contraceptive use and vulvar condyloma development
♦
The lesions may be associated with or progress to high-grade vulvar intraepithelial neoplasia (VIN) or squamous cell carcinoma (SCC), especially in immunosuppressed women, often due to coexisting high-risk HPV
♦
The lesions are self-limited in many cases. If increasing in size or numbers, patients can be treated with cryotherapy, surgery, or podophyllin resin
Microscopic
♦
Architecturally, condylomas are characterized by simple to complex branching papillae composed of acanthotic squamous epithelium and fibrovascular cores (Fig. 30.5A). Sometimes, endophytic or downward growth of rete pegs is seen
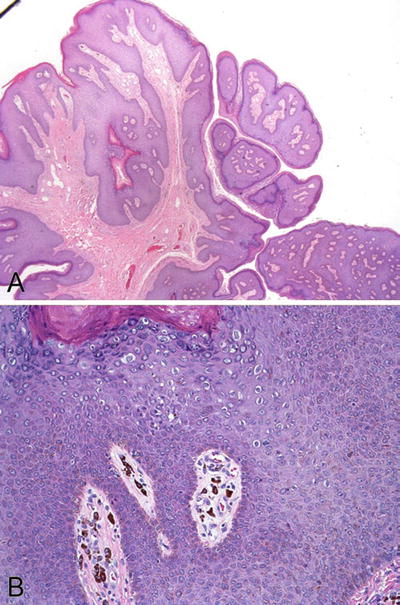

Fig. 30.5.
Condyloma acuminatum . (A) Typical complex branching papillae are composed of acanthotic squamous epithelium and fibrovascular cores. (B) Koilocytes are present in the squamous epithelium.
♦
The diagnostic feature of condylomas is the presence of koilocytes in the superficial layers (Fig. 30.5B). Koilocytes are HPV-infected keratinocytes that have atypical nuclei with a perinuclear clear zone (halo) of clear cytoplasm. The atypical nuclei have three features including enlargement, hyperchromasia or coarse chromatin, and irregular nuclear membrane
♦
Binucleated or multinucleated koilocytes are commonly seen. The koilocytes may vary in size and shape
♦
MIB1 or Ki67 expression is present in the upper two-thirds of the squamous epithelium. p16 immunoreactivity is focal, cytoplasmic, and confined to the upper half of the epithelium. These staining pattern may aid the diagnosis when unequivocal diagnostic features are absent
♦
Variants of condyloma include flat condyloma (more in the cervix), seborrheic keratosis-like condyloma, and condyloma with pseudobowenoid change (prominent apoptosis in the superficial keratinocytes)
♦
Some authorities group condylomas under low-grade VIN (VIN1). Attention should be paid to the presence of any high-grade VINs (see “High-Grade Squamous Intraepithelial Lesion”) when you examine the condylomas
Differential Diagnosis
♦
Verrucous carcinomas sometimes are referred to as “giant condylomas.” These tumors are usually large and solitary and often HPV negative and lack the fine branching papillae and koilocytosis of condylomas. Patients with verrucous carcinoma are usually older (see “Premalignant and Malignant Epithelial Tumors”)
♦
Warty VINs have significant degrees of nuclear atypia and more obvious mitosis in at least the lower one-third of the epithelium. p16 immunostain is strong and diffuse
♦
Squamous vestibular papillomatosis confines to the vestibular area. The papillae are smaller and lack koilocytosis and hyperkeratosis (see “Benign Tumors”)
♦
Condyloma lata is a secondary lesion of syphilis (see below)
Candidiasis
Clinical
♦
Vulvar candidiasis is often an external manifestation of a vaginal infection by Candida
♦
Patients commonly present with moist, red lesions with intensive pruritus
♦
Diagnosis is usually made by skin scraping. Biopsy is rarely needed
Microscopic
♦
Identification of fungal hyphae within the keratin or superficial epithelium by GMS or PAS stains in a background of acute inflammation is diagnostic of fungal infection
Molluscum Contagiosum
Clinical
♦
Molluscum contagiosum is a contagious viral disease, transmitted through intimate or sexual contact. It is more commonly seen in immunosuppressed patients. The lesions are usually asymptomatic, but can become pruritic
♦
The causative agent is a DNA poxvirus
♦
The characteristic lesions are multiple, small and smooth papules (3–6 mm diameter) with a central punctum or umbilication (Fig. 30.6A)
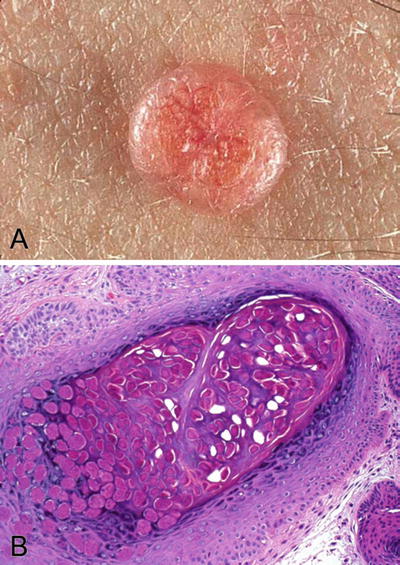

Fig. 30.6.
Molluscum contagiosum . (A) A small smooth papule with a central punctum is a typical gross appearance. (B) Molluscum bodies consist of homogeneous intracytoplasmic DNA virus inclusions.
♦
It is usually diagnosed clinically without biopsy. No therapy is needed
Microscopic
♦
Marked acanthosis and presence of characteristic intracytoplasmic viral inclusions (molluscum bodies or Henderson–Paterson bodies) are the main histologic features of a typical lesion (Fig. 30.6B)
Herpesvirus Infection
Clinical
♦
The causative agent is herpes simplex virus (HSV), predominantly type 2
♦
Dysuria or urinary retention with vulvar pain is the initial presentation
♦
Systemic symptoms with fever and inguinal lymphadenopathy are present in about two-thirds of the patients
♦
Vesicles, pustules, and painful shallow ulcers appear sequentially, usually within a week of infection. The acute ulcers persist for 2–6 weeks (average 19 days) and then heal without scarring
♦
Acyclovir is the main therapy given early in the course of illness
Microscopic
♦
The HSV-infected epithelial cells are characterized by “ground-glass” appearance of the nuclei or eosinophilic intranuclear inclusions at the periphery of the vesicles or ulcers (Fig. 30.7)
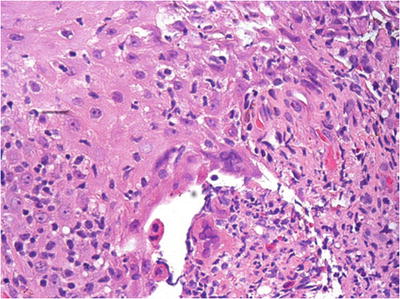

Fig. 30.7.
Herpes simplex virus of vulva . Ulceration with a marked acute and chronic inflammation is evident. Multinucleation with typical ground-glass appearance is noted.
♦
Tzank preparation of a fresh lesion is an effective method for diagnosis
♦
Anti-HSV can be used to aid the diagnosis in certain clinical settings
Cytomegalovirus Infection
♦
Cytomegalovirus (CMV) infection presents with vulvar ulcer, most commonly seen in HIV infected women
♦
The characteristic CMV inclusion bodies are both intranuclear and intracytoplasmic, involving epithelial cells and vascular endothelial cells
Syphilis
Clinical
♦
Syphilis is a venereal disease caused by the spirochaete Treponema pallidum
♦
Chancre is the primary lesion and occurs within days or months of initial sexual contact. A typical chancre appears as a painless, indurated, shallow, and clean-based ulcer with raised edges. Chancre can be single or multiple and usually heals within 2–6 weeks without leaving a scar
♦
The secondary phase becomes evident within several months. It is characterized by rashes of mucous membranes and skin in the palms of the hands and soles of the feet. The characteristic lesion in the vulva is condyloma lata, which is an elevated plaque of several centimeters in diameter
♦
The tertiary gumma is rarely seen on vulva
Microscopic
♦
A large quantity of perivascular plasma cell infiltrate is characteristically seen in both chancre and condyloma lata (Fig. 30.8)
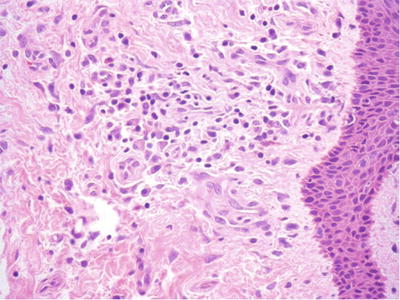

Fig. 30.8.
Condyloma lata . Stromal fibrosis, squamous hyperplasia, and chronic inflammation with a perivascular plasma cell-rich component may be seen.
♦
Condyloma lata have marked acanthosis, hyperkeratosis, and papillomatosis with some neutrophils in the epidermis
♦
Warthin–Starry stain, dark-field examination, or immunofluorescence stain can be used to demonstrate the spirochetes in these lesions
Lymphogranuloma Venereum
♦
Lymphogranuloma venereum is caused by Chlamydia trachomatis
♦
It is more common in the tropical and subtropical regions and rarely seen in the United States
♦
Patients typically present with nontender skin ulcers, followed by painful inguinal lymphadenitis. Chronic lymphatic obstruction occurs in later phase of the infection leading to vaginal and rectal fibrosis
♦
Chronic inflammation is extensive, but not specific
♦
Diagnosis is relied on the characteristic clinical findings, culture, and complement fixation tests
Cystic Lesions
Bartholin Duct Cyst and Abscess
Clinical
♦
Bartholin duct cyst accounts for a majority of the symptomatic vulvar cysts in a gynecologic clinic. It occurs secondary to obstruction of the vestibular orifice of Bartholin ducts and accumulation of secretions from the glands
♦
Bartholin abscess occurs as a result of acute infection of the Bartholin glands
♦
An average size of the cyst or abscess is 1–2 cm but can be much larger
♦
Recurrent cysts or a palpable mass after cyst drainage in postmenopausal women may require excision to rule out malignancy of the Bartholin gland
Microscopic
♦
Bartholin cysts are lined by squamous, transitional, mucinous, ciliated, or flattened nonspecific epithelium
♦
A typical location and the presence of normal Bartholin glands adjacent to the cyst facilitate distinction from cysts arising in minor vestibule glands (Fig. 30.9)
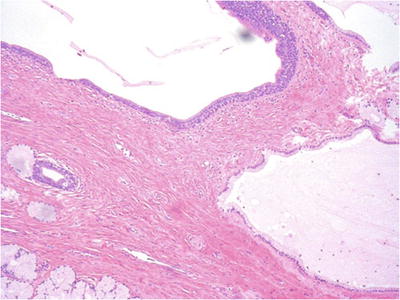

Fig. 30.9.
Bartholin gland cyst. The cyst is lined by cuboidal mucinous and transitional or indifferent cells. Atrophic changes are seen in adjacent Bartholin glands.
Mucinous and Ciliated Vestibular Cysts
♦
These cysts are rare and arise from the minor vestibular glands. History of 5-fluorouracil therapy for condyloma is present in some cases
♦
Cysts are lined by columnar mucinous epithelium, ciliated nonmucinous epithelium, or a combination of the two. No myoepithelial cell layer or outer smooth muscle layer is present
♦
Surgical excision is the management
Epidermal Inclusion Cyst
♦
They are commonly seen in the labia majora and may result from obstruction of the pilosebaceous ducts and glands
♦
Patients may present with complaints of a palpable nodularity within the vulva
♦
Cyst is lined by stratified cornified squamous epithelium and filled with keratinaceous debris
Other Cysts
♦
Other cysts, more commonly seen in the vagina, can rarely occur in vulva and include mesothelial cyst , Skene duct cyst , and mesonephric-like cyst (see “Vagina”)
Benign Discolored Lesions
Lichen Sclerosus
Clinical
♦
Lichen sclerosus accounts for 30–40% of nonneoplastic epithelial lesions of the vulva. Middle-aged and elderly women are more commonly affected than younger women. A similar condition in male patients is called balanitis xerotica obliterans
♦
This condition is probably related to chronic lymphocyte-mediated process. But the exact etiology is unclear. Proposed mechanisms include immunologic, genetic, and hormonal abnormalities. Deficiencies of androgen receptor and epidermal growth factor were reported in some studies
♦
Typical lesions of vulvar lichen sclerosus are irregular, ill-defined hypopigmented patches, often multiple, bilateral, and sometimes symmetrical. In advanced stages, the affected skin is shiny and wrinkled (“cigarette paper”); the labia are atrophic, and the introitus is narrowed (Fig. 30.10A)
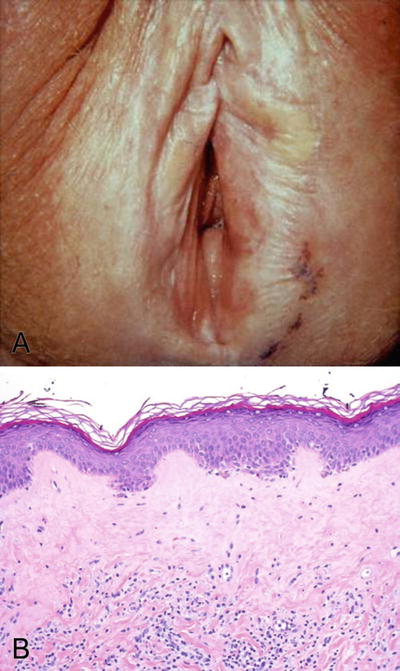

Fig. 30.10.
Lichen sclerosis . (A) Gross appearance of lichen sclerosis at an advanced stage. (B) Epidermal atrophy, loss of rete ridges, and hyalinization in the upper dermis are the typical histologic features.
♦
Growing evidence suggests that lichen sclerosus is a premalignant lesion. 1–5% of cases of lichen sclerosus progress to vulvar SCC. More than 60% of well-differentiated VIN and invasive keratinizing squamous cell carcinomas are associated with synchronous lichen sclerosus
Microscopic
♦
The characteristic features include atrophic epidermis with loss of rete ridges and a zone of homogeneous subepithelial hyalinization or edema (Fig. 30.10B)
♦
There is a loss of elastic fibers and vasculature
♦
A band of chronic lymphocytic infiltration may be present in the superficial dermis
♦
Early lichen sclerosus may show markedly acanthosis, luminal hyperkeratosis, and hyperkeratosis. A thickened basement membrane may be appreciable
♦
Lichen sclerosus can be superimposed with lichen simplex chronicus or squamous cell hyperplasia (see below)
♦
Lichen sclerosus associated with invasive squamous cell carcinoma may show basal atypia and positive p53 stain, with a discontinuous pattern. p16 staining is negative
Squamous Cell Hyperplasia
♦
Squamous cell hyperplasia is a descriptive term applicable, both clinically and histologically, to epithelial thickening of the vulva without identifiable causes or with difficulties in classification (hyperplastic dystrophy as old terminology)
♦
Localized pruritus is the most common presenting symptom Unilateral irregular white plaque-like lesions may be identified
♦
Nonspecific thickening of the vulvar epithelium is demonstrated by acanthosis with elongation, widening, and deepening of the rete ridges. Hyperkeratosis or parakeratosis may be present. Cellular atypia, dermal inflammation, and fibrosis are absent (Fig. 30.11)


Fig. 30.11.
Squamous cell hyperplasia . Epidermis shows prominent acanthosis with elongated rete pegs. No dysplasia or nuclear atypia is present.
♦
If significant inflammation is present, a diagnosis of lichen simplex chronicus should be considered (see “Inflammatory Lesions”)
♦
If cellular atypia is present, the lesion should be classified as differentiated VIN. When lichen sclerosus or squamous cell hyperplasia is associated with VIN, both diagnoses should be reported
♦
Squamous cell hyperplasia are found adjacent to up to 40% of squamous cell carcinoma and should be monitored clinically
Vulvar Acanthosis with Altered Differentiation
♦
Vulvar acanthosis with altered differentiation is a distinctive squamous proliferation that often seen adjacent to verrucous carcinoma
♦
It is characterized by marked acanthosis with variable verruciform architecture, loss of the granular layer, superficial epithelial cell pallor, and multilayered parakeratosis (plaque-like)
♦
The lesion may be a precursor or risk factor for verrucous carcinoma
Seborrheic Keratosis
♦
Seborrheic keratosis is a raised and pigmented warty lesion occurring on the hair-bearing areas of the vulva
♦
Seborrheic keratosis may be single or multiple and can form clusters
♦
Microscopically, it consists of a basaloid cell proliferation with acanthosis, papillomatosis, hyperkeratosis, and epithelial invaginations forming horn cysts (Fig. 30.12)
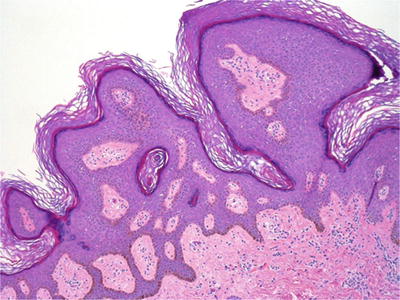

Fig. 30.12.
Seborrheic keratosis with a horn cyst.
♦
Increased melanin pigment in basal and parabasal layers may be seen in the lesion
♦
Multiple seborrheic keratosis presenting over a short period of time may be associated with internal malignancy (referred to as Leser–Trelat syndrome )
Lentigo Simplex and Melanosis
♦
These hyperpigmented macular lesions result from a localized excessive production of melanin by melanocytes. They typically occur in white women of reproductive age
♦
Microscopically, the lesions show basilar keratinocytic hyperpigmentation and melanocytic hyperplasia without nesting or atypia. Epidermal hyperplasia is usually seen in lentigo simplex (Fig. 30.13)
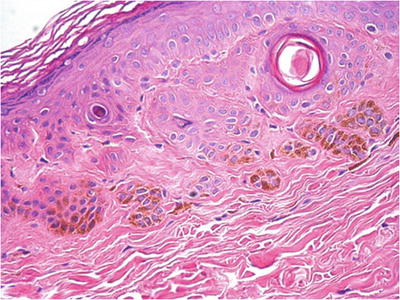

Fig. 30.13.
Lentigo simplex or melanosis (depending on the size).
♦
There are no distinctive histologic features to differentiate lentigo simplex from melanosis other than size of the pigmented area (arbitrarily <4 mm for lentigo simplex)
♦
Both conditions usually have no clinical significance
♦
The presence of atypical melanocytes, especially in nests and in all layers of the epidermis, warrants a diagnosis of malignant melanoma in situ (see “Malignant Nonepithelial Tumors”)
Melanocytic Nevi
♦
Vulvar melanocytic nevi are much less common than those occurring in extravulvar sites
♦
Most vulvar nevi are either compound or intradermal in type. Pure junctional type is rare on the vulva
♦
Atypical melanocytic nevi of genital type (AMNGT) refers to a small percentage of vulvar nevi which shows distinctive, atypical features that differ from those of the usual dysplastic nevus
♦
AMNGT are typically symmetrical on cross sections and show maturation of the melanocytes in the dermis. They usually lack significant pagetoid spread and mitosis
♦
Differential diagnosis of AMNGT includes dysplastic nevi and superficial spreading melanoma (see “Malignant Nonepithelial Tumors”). Please read a comprehensive chapter by Clark et al., if any concerns of the differential diagnosis arise
♦
The vast majority of vulvar nevi require no therapy. However, concerns regarding the malignant potential results in excision in most cases
Benign Tumors
Fibroepithelial Stromal Polyp
♦
Fibroepithelial stromal polyp (acrochordon or skin tag) is a benign polypoid mass, most commonly occurs in vagina, and is relatively uncommon on the hair-bearing skin of the vulva
♦
These tumors are soft and fleshy and may be pigmented
♦
A typical polyp consists of a fibrovascular stalk covered by a stratified keratinizing squamous epithelium
♦
Stroma within the stalk may be edematous and hypocellular. Stromal cells are bland and spindle shaped with delicate uni- or bipolar cytoplasmic processes. Occasionally, some stromal cells may have significant nuclear pleomorphism with atypia, particularly when patient is pregnant (Fig. 30.14)
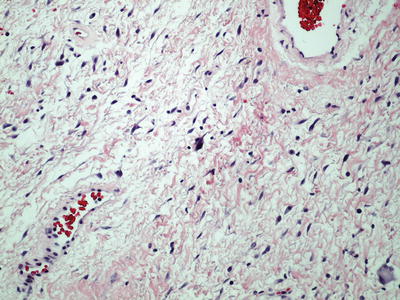

Fig. 30.14.
Fibroepithelial stromal polyp with atypical stromal cells.
Squamous Vestibular Papillomatosis
♦
Squamous vestibular papillomatosis refers to multiple to numerous, small papillary projections in the medial aspect of the labia minora and in the vestibule exterior to the hymenal ring. Each papilloma is about 1–2 mm in diameter
♦
This condition occurs almost exclusively in women of reproductive age
♦
There is no causal relationship between HPV infection and squamous vestibular papillomatosis
♦
Presenting symptoms include pruritus, burning sensation, and dyspareunia. Some women may be asymptomatic
♦
Histologic features are papillary fronds of a plain, nonkeratinized, glycogenated squamous epithelium with an underlying proliferation of capillaries in the dermal papillae. The key feature is absence of koilocytes or nuclear atypia in the glycogenated epithelium, differentiating from condyloma
Keratoacanthoma
♦
These tumors are neoplasms of keratinocytes arising from follicular epithelium. They arise rapidly, often in the areas associated with prior trauma
♦
They are discrete and inverted and form a central keratin-filled crater with focal infiltration at its superficial dermis, resembling pseudoepitheliomatous hyperplasia
♦
Most tumors are self-limited. Similar tumors with atypia and metastasis have been reported, and in this scenario, a diagnosis of SCC, keratoacanthoma type, would be more appropriate
Papillary Hidradenoma (Hidradenoma Papilliferum)
Clinical
♦
Papillary hidradenoma is the most common benign glandular tumor of the vulva and arises from a specialized anogenital sweat gland or mammary-like glands
♦
It usually presents as a painless nodule or cyst <2 cm in diameter in women of reproductive age
♦
The most common location is interlabial sulcus
Microscopic
♦
The tumor is composed of complex proliferation of branching papillae, tubules, and cysts lined by a double layer of cells (columnar epithelial cells of the inner layer and myoepithelial cells in the outer layer, Fig. 30.15)
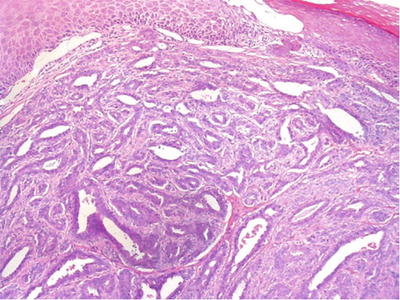

Fig. 30.15.
Papillary hidradenoma (hidradenoma papilliferum).
♦
The epithelial cells are well defined and have an apocrine appearance. They may exhibit mild atypia and stratification and have occasional mitotic figures
♦
Malignant transformation to adenocarcinoma is extremely rare
♦
Differential diagnosis includes intraductal papillomas arising in ectopic breast tissue. Presence of benign ectopic breast tissue adjacent to the tumor favors the latter
Granular Cell Tumor
Clinical
♦
Granular cell tumor is probably of peripheral nerve sheath origin. Between 5% and 15% of granular cell tumors arise in the vulva
♦
The patients are usually of reproductive or postmenopausal age. They typically present with a solitary or rarely with several subcutaneous nontender nodules in the labia majora or in the vicinity of the clitoris
♦
Multicentric and familial tumors have been reported
♦
The tumors are almost always clinically benign
Microscopic
♦
A typical tumor is composed of nests and sheets of large polygonal cells with eosinophilic granular cytoplasm and bland small nuclei (Fig. 30.16A). Tumor cells are intermingled with strands of collagen and occasional chronic inflammatory cells
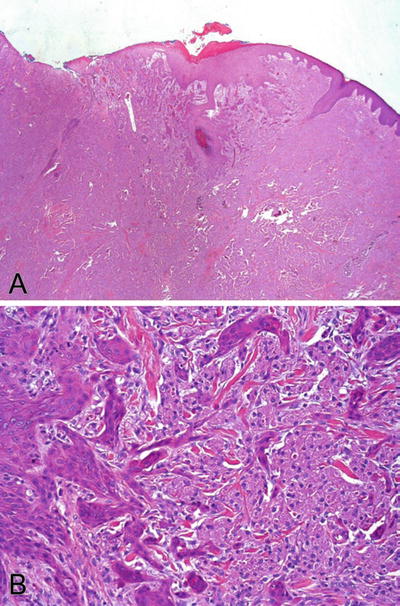

Fig. 30.16.
Granular cell tumor . (A) The tumor is localized in the dermis. The tumor cells are large and contain coarsely granular and eosinophilic cytoplasm. The nuclei are uniformly bland. The epidermis shows pseudoepitheliomatous hyperplasia. The pattern could be misdiagnosed as invasive squamous cell carcinoma. An area from (A) is magnified in (B) to appreciate the pseudoinvasive pattern.
♦
Pseudoepitheliomatous hyperplasia of the overlying squamous epithelium is a striking feature (Fig. 30.16B). This can be confused with a well-differentiated squamous cell carcinoma, especially in a superficial biopsy specimen
♦
PAS, S-100, and myelin basic protein stains are positive in the granular cytoplasm
Angiokeratoma
Clinical
♦
Angiokeratomas are vascular lesions and occurs almost exclusively on vulva of the child-bearing women
♦
The cause is unknown, but a patient with multiple angiokeratomas on the vulva and other sites should receive consultation for Fabry disease, which is an X-linked recessive disease associated with a deficiency of galactosidase A
♦
Patients are usually asymptomatic. The lesions are small, 2–5 mm, red to purple papules
Microscopic
♦
The tumors are characterized by prominent endothelial-lined blood vessels immediately beneath the basement membrane of the overlying epithelium and separated by the rete ridges
♦
The rete ridges form epithelial cords, which separate the vascular channels, resulting in a multilocular appearance of the lesions (Fig. 30.17)
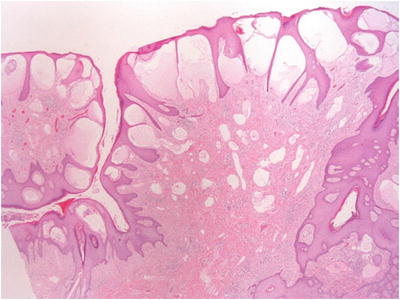

Fig. 30.17.
Angiokeratoma of Fordyce .
Aggressive Angiomyxoma
Clinical
♦
Aggressive angiomyxoma is synonymously referred to as deep angiomyxoma
♦
It is a locally infiltrative tumor of the deep soft tissue of the pelvic–perineal region
♦
AA mainly occurs in women in the third to fourth decades
♦
Typical presentation is a large, often greater than 10 cm in size, slow-growing, and painless mass that is often thought to be Bartholin gland cyst clinically
♦
The masses are often substantially larger on image studies than clinically suspected
♦
Symptoms are usually vague and may have slightly increased pressure in the urogenital or anorectal areas. Rapid growth may occur during pregnancy
♦
These tumors are indolent but locally infiltrative. Recurrence rate is high, but distant metastasis generally does not happen
♦
Hormonal therapy with gonadotropin-releasing hormone (GnRH) agonist may be effective in reducing the tumor size
Microscopic
♦
The tumors are paucicellular and composed of fibroblasts, myofibroblasts, and numerous characteristically thin- and thick-walled vessels in an abundant myxoid matrix (Fig. 30.18). Thick vessels may have hyalinized or muscle wall. Perivascular cuffs of collagen and bundles of smooth muscle are common
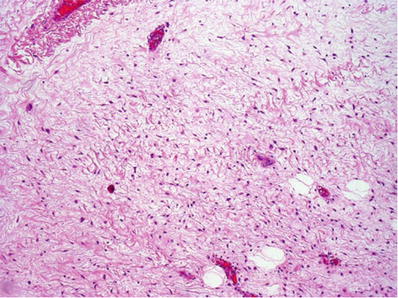

Fig. 30.18.
Aggressive angiomyxoma with bland spindle-shaped cells in a hypocellular myxoid stroma containing a mixture of thick- and thin-walled blood vessels.
♦
Multinucleated cells may be present. Mitosis is absent
♦
Local infiltration of surrounding soft tissue is present, often with entrapment of skeletal muscle and nerves
♦
Extravasated erythrocytes are common
♦
The tumor cells are immunoreactive for desmin, CD34, CD44, smooth muscle actin, ER, and PR but negative for S-100. HMGA2 nuclear immunoreactivity is present in most cases
Differential Diagnosis
♦
Differential diagnosis includes other myxoid lesions such as superficial angiomyxoma (cutaneous myxoma), myxoid neurofibroma, myxoid liposarcoma, myxoid smooth muscle tumors, and myxoid malignant fibrous histiocytoma
♦
Superficial location, small size, circumscription, absence of thick-walled vessels, nonreactivity for desmin and ER/PR, and occasional association with Carney complex facilitate a diagnosis of superficial angiomyxoma
♦
Distinctive vascular pattern is not present in other myxoid tumors
Angiomyofibroblastoma
♦
Angiomyofibroblastoma occurs almost exclusively in the vulvovaginal region of middle-aged women, presenting as a slow-growing, well-circumscribed, painless nodule and mostly <5 cm in greatest dimension
♦
Characteristic histologic features are alternating hypercellular and hypocellular zones with numerous irregularly distributed small to medium, thin-walled, arborizing vessels, often with perivascular fibrosis
♦
The tumor cells have variable appearance (spindled, ovoid, plasmacytoid, or epithelioid) and separated by wavy strands or thick bundles of collagen (Fig. 30.19). They tend to concentrate around the vessels in the hypercellular zone
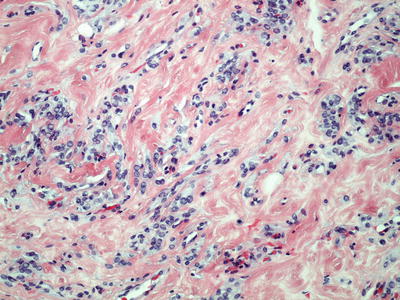

Fig. 30.19.
Angiomyofibroblastoma with small groups of epithelioid or spindle-shaped cells and capillary-like vascular channels.
♦
Most nuclei are bland. Occasional cells are binucleated or multinucleated. The plasmacytoid cells have eccentric nuclei. Mitotic features are absent or rare
♦




The tumor cells are positive for vimentin, desmin, ER, and PR, rarely positive for actin and CD34, and negative for S-100 or keratin
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


