Chapter 10 Viruses
Viruses can replicate only in a host cell
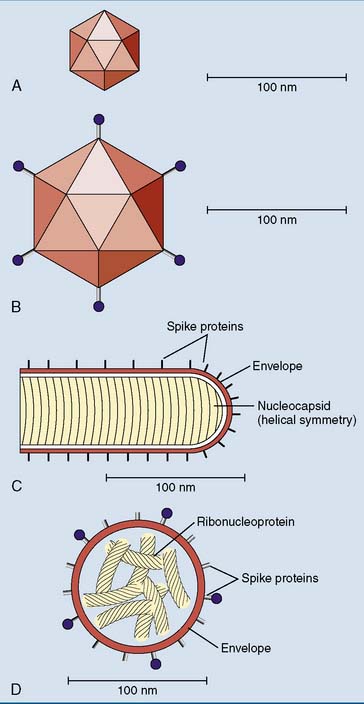
Many animal viruses are enclosed by an envelope, which is a piece of host cell membrane appropriated by the virus while it is budding out of its host cell. The envelope is studded with viral proteins, the spike proteins (Fig. 10.1).
Bacteriophage T4 destroys its host cell
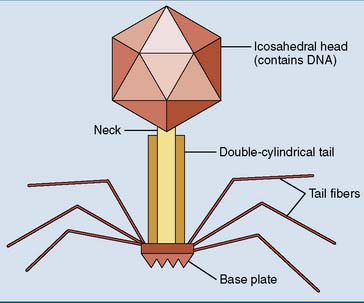
Viruses that infect bacteria are called bacteriophages (“bacteria eaters”) or simply “phages.” Bacteriophage T4 is a classic example. It is one of the most complex viruses known (Fig. 10.2), with a double-stranded DNA genome of about 150 genes tightly packed into the head portion of the virus particle. Attached to the head is a short neck followed by a cylindrical tail with two coaxial hollow tubes, a base plate, and six spidery tail fibers. This complex capsid consists of about 40 virus-encoded polypeptides, each present in many copies.
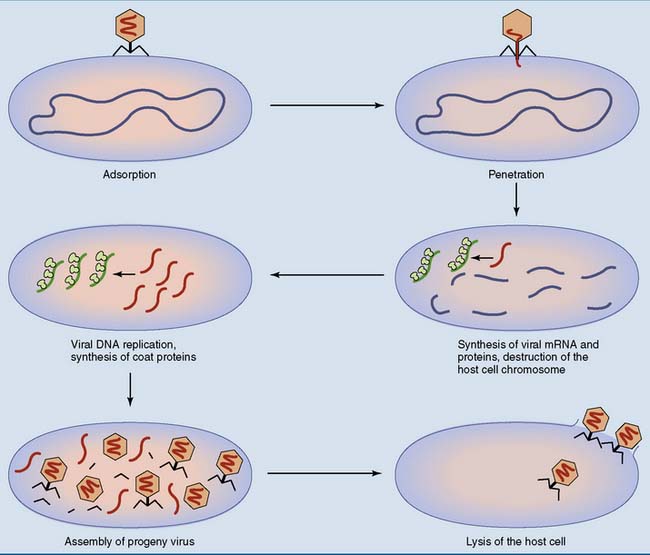
T4 is constructed like a syringe that injects its DNA into the host cell. First, the tail fibers bind to a component of the bacterial cell wall that serves as a virus receptor. Next, the sheath of the tail contracts, its inner core penetrates the cell wall, and the viral DNA is injected into the cell. Only the DNA enters the host cell. The protein coat remains outside (Fig. 10.3).
DNA viruses substitute their own DNA for the host cell DNA
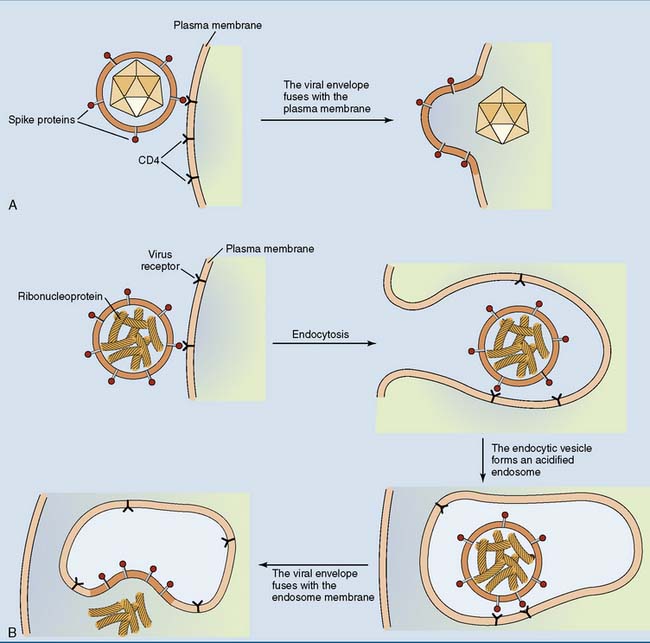
Some enveloped viruses fuse their envelope with the plasma membrane of the host cell, whereas others trigger their own endocytosis (Fig. 10.4).
λ Phage can integrate its DNA into the host cell chromosome
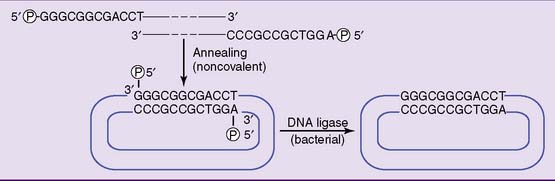
Like T4 phage, λ phage is constructed as a syringe that injects its DNA into the host cell. Its genome, with about 50 genes, is a linear double-stranded DNA molecule of 48,502 base pairs with single-stranded ends of 12 nucleotides each. These single-stranded overhangs have complementary base sequences. They anneal (base pair) as soon as the viral DNA enters the host cell, and the viral genome is linked into a circle by bacterial DNA ligase (Fig. 10.5). Lytic infection can now proceed as described previously for T4 phage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree