INTRODUCTION
Vibrio, Campylobacter, and Helicobacter are Gram-negative rods that are all widely distributed in nature. The vibrios are found in marine and surface waters. The campylobacters are found in many species of animals, including many domesticated animals. Vibrio cholerae produces an enterotoxin that causes cholera, a profuse watery diarrhea that can rapidly lead to dehydration and death. Campylobacter jejuni is a common cause of enteritis in humans. Helicobacter pylori is associated with gastritis and duodenal ulcer disease.
THE VIBRIOS
Vibrios are among the most common bacteria in surface waters worldwide. They are curved aerobic rods and are motile, possessing a polar flagellum. V cholerae serogroups O1 and O139 cause cholera in humans, and other vibrios may cause skin and soft tissue infections, sepsis, or enteritis. The medically important vibrios are listed in Table 17-1.
| Organism | Human Disease |
|---|---|
| Vibrio cholerae serogroups O1 and O139 | Epidemic and pandemic cholera |
| Vibrio cholerae serogroups non-O1/non-O139 | Cholera-like diarrhea; mild diarrhea; rarely, extraintestinal infection |
| Vibrio parahaemolyticus | Gastroenteritis, wound infections, septicemia |
| Vibrio vulnificus | Gastroenteritis, wound infections, septicemia |
VIBRIO CHOLERAE
The epidemiology of cholera closely parallels the recognition of V cholerae transmission in water and the development of sanitary water systems.
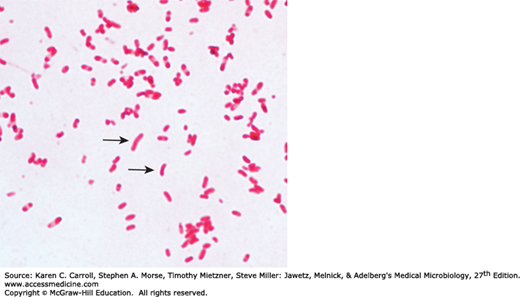
Upon first isolation, V cholerae is a comma-shaped, curved rod 2–4 μm long (Figure 17-1). It is actively motile by means of a polar flagellum. On prolonged cultivation, vibrios may become straight rods that resemble the Gram-negative enteric bacteria.
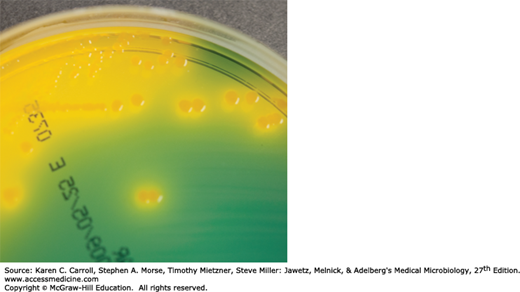
V cholerae produces convex, smooth, round colonies that are opaque and granular in transmitted light. V cholerae and most other vibrios grow well at 37°C on many kinds of media, including defined media containing mineral salts and asparagine as sources of carbon and nitrogen. V cholerae grows well on thiosulfate-citrate-bile-sucrose (TCBS) agar, a media selective for vibrios, on which it produces yellow colonies (sucrose fermented) that are readily visible against the dark-green background of the agar (Figure 17-2). Vibrios are oxidase positive, which differentiates them from enteric Gram-negative bacteria. Characteristically, vibrios grow at a very high pH (8.5–9.5) and are rapidly killed by acid. Cultures containing fermentable carbohydrates therefore quickly become sterile.
In areas where cholera is endemic, direct cultures of stool on selective media, such as TCBS, and enrichment cultures in alkaline peptone water are appropriate. However, routine stool cultures on special media such as TCBS generally are not necessary or cost effective in areas where cholera is rare.
V cholerae regularly ferments sucrose and mannose but not arabinose. A positive oxidase test result is a key step in the preliminary identification of V cholerae and other vibrios. Most Vibrio species are halotolerant, and NaCl often stimulates their growth. Some vibrios are halophilic, requiring the presence of NaCl to grow.
Many vibrios share a single heat-labile flagellar H antigen. Antibodies to the H antigen are probably not involved in the protection of susceptible hosts.
V cholerae has O lipopolysaccharides that confer serologic specificity. There are at least 206 O antigen groups. V cholerae strains of O group 1 and O group 139 cause classic cholera; occasionally, non-O1/non-O139 V cholerae causes cholera-like disease. Antibodies to the O antigens tend to protect laboratory animals against infections with V cholerae.
The V cholerae serogroup O1 antigen has determinants that make possible further typing; the serotypes are Ogawa, Inaba, and Hikojima. Two biotypes of epidemic V cholerae have been defined, classic and El Tor. The El Tor biotype produces a hemolysin, gives positive results on the Voges-Proskauer test, and is resistant to polymyxin B. Molecular techniques can also be used to type V cholerae. Typing is used for epidemiologic studies, and tests generally are done only in reference laboratories.
V cholerae O139 is very similar to V cholerae O1 El Tor biotype. V cholerae O139 does not produce the O1 lipopolysaccharide and does not have all the genes necessary to make this antigen. V cholerae O139 makes a polysaccharide capsule like other non-O1 V cholerae strains, but V cholerae O1 does not make a capsule.
V cholerae produce a heat-labile enterotoxin with a molecular weight (MW) of about 84,000, consisting of subunits A (MW, 28,000) and B (see Chapter 9). Ganglioside GM1 serves as the mucosal receptor for subunit B, which promotes entry of subunit A into the cell. Activation of subunit A1 yields increased levels of intracellular cyclic adenosine monophosphate (cAMP) and results in prolonged hypersecretion of water and electrolytes. There is increased sodium-dependent chloride secretion, and absorption of sodium and chloride by the microvilli is inhibited. Electrolyte-rich diarrhea occurs—as much as 20–30 L/day—with resulting dehydration, shock, acidosis, and death. The genes for V cholerae enterotoxin are on the bacterial chromosome. Cholera enterotoxin is antigenically related to LT of Escherichia coli and can stimulate the production of neutralizing antibodies. However, the precise role of antitoxic and antibacterial antibodies in protection against cholera is not clear.
Under natural conditions, V cholerae is pathogenic only for humans. A person with normal gastric acidity may have to ingest as many as 1010 or more V cholerae to become infected when the vehicle is water because the organisms are susceptible to acid. When the vehicle is food, as few as 102–104 organisms are necessary because of the buffering capacity of food. Any medication or condition that decreases stomach acidity makes a person more susceptible to infection with V cholerae.
Cholera is not an invasive infection. The organisms do not reach the bloodstream but remain within the intestinal tract. Virulent V cholerae organisms attach to the microvilli of the brush border of epithelial cells. There they multiply and liberate cholera toxin and perhaps mucinases and endotoxin.
About 50% of infections with classic V cholerae are asymptomatic, as are about 75% of infections with the El Tor biotype. The incubation period is 12 hours–3 days for persons who develop symptoms, depending largely on the size of the inoculum ingested. There is a sudden onset of nausea and vomiting and profuse diarrhea with abdominal cramps. Stools, which resemble “rice water,” contain mucus, epithelial cells, and large numbers of vibrios. There is rapid loss of fluid and electrolytes, which leads to profound dehydration, circulatory collapse, and anuria. The mortality rate without treatment is between 25% and 50%. The diagnosis of a full-blown case of cholera presents no problem in the presence of an epidemic. However, sporadic or mild cases are not readily differentiated from other diarrheal diseases. The El Tor biotype tends to cause milder disease than the classic biotype.
Specimens for culture consist of mucus flecks from stools.
The microscopic appearance of smears made from stool samples is not distinctive. Dark-field or phase contrast microscopy may show the rapidly motile vibrios.
Growth is rapid in peptone agar, on blood agar with a pH near 9.0, or on TCBS agar, and typical colonies can be picked in 18 hours. For enrichment, a few drops of stool can be incubated for 6–8 hours in taurocholate peptone broth (pH, 8.0–9.0); organisms from this culture can be stained or subcultured. Accurate identification of vibrios, including V cholerae, using commercial systems and kit assays is quite variable.
V cholerae organisms are further identified by slide agglutination tests using anti-O group 1 or group 139 antisera and by biochemical reaction patterns. The diagnosis of cholera under field conditions has been reported to be facilitated by a sensitive and specific immunochromatographic dipstick test.
Gastric acid provides some protection against cholera vibrios.
An attack of cholera is followed by immunity to reinfection, but the duration and degree of immunity are not known. In experimental animals, specific IgA antibodies occur in the lumen of the intestine. Similar antibodies in serum develop after infection but last only a few months. Vibriocidal antibodies in serum (titer ≥1:20) have been associated with protection against colonization and disease. The presence of antitoxin antibodies has not been associated with protection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree