Ventral Abdominal Hernia
Guy R. Voeller
While a lot has occurred in the world of herniology since the last edition of Mastery of Surgery in 2007, many things remain the same. High recurrence rates remain a problem. The Netherlands group published the long-term results of their randomized, prospective trial looking at suture repair versus mesh repair for incisional hernias (IH) 6 cm or less. The 10-year recurrence rate even with mesh was 32% versus 63% for suture repair. The technique for mesh placement in this study was not ideal and may explain the 32% recurrence. Flum in 2003 reported a retrospective population-based cohort study on IH from 1987 to 1999 in Washington State. He found that while the use of mesh was associated with a 24% decrease in the chance of having another repair over the short term, the use of mesh may simply be delaying the appearance of the recurrence. This may again point to the importance of proper placement of the mesh, with the sublay method (to be described) becoming more prominent and well known during the later part of this study. There are new biomaterials and new techniques available and we have also learned that some things may not be as good as we thought when it comes to long-term success for ventral hernia repair.
Biomaterials
The development of laparoscopic repair for ventral hernias required that the mesh be placed in contact with the viscera. This led to a literal explosion of companies bringing new meshes to market very quickly. The Food and Drug Administration (FDA) is very lenient when it comes to requiring any clinical studies prior to these meshes being introduced (as opposed to drugs) so it is critical that the surgeon understand the pluses and minuses of these different materials since their patients are the “guinea pigs” so to speak. While at one time not too long ago surgeons simply had to choose from heavy-weight polypropylene or polyester they are now confronted with many more choices. With cost being an issue and competition between companies intense, the surgeon is often caught in the middle between the companies and the hospital to make a choice of what meshes to have available. Knowledge of these products is critical.
Synthetic Meshes
The three main meshes still available are polypropylene (PPM), polyester, and polytetrafluoroethylene (ePTFE) and various combinations of these materials. The movement to lighter-weight or less dense PPM began in the 1990s when Schumpelick’s group showed that dense PPM stiffened and contracted and that this may be the cause of chronic pain and stiffness of the abdominal wall that some people experience. Ramshaw and the group at Missouri have now taken the next step and are evaluating mesh explants. They have developed a cleaning process to remove adherent tissue without changing the characteristics of the mesh product. They evaluated hundreds of explants and found that as a result of the chronic inflammatory response the mesh is subjected to oxidation. This leads to cracking, changes in thermal properties, weight loss, loss of compliance, and other changes. The explanted material is significantly different from the pristine new product due to oxidation and this, they believe, can explain the problems that surgeons experience clinically. The clinical and experimental work has led to the development of many less dense or what is called lightweight PPM. Many believe that these should be the “new” standard of care when mesh is used and in fact Cobb and Heniford believe that lightweight meshes provide more than adequate strength for repair while resulting in less stiffness and restriction of the abdominal wall. This should be tempered somewhat by a 2005 study of Conze and others who performed a prospective randomized trial of lightweight PPM versus polyester versus regular PPM placed in a sublay fashion as described by Rives. They evaluated the patients out to 2 years and found that complications were the same and the SF-36 scores (assessing physical function and daily activities) were the same between groups. The one thing that was different was that the recurrence rate in the lightweight group
was 17% versus only 7% for the standard meshes. This may have been due to the technique since 19 of the 20 recurrences had absorbable suture used for mesh fixation and three of the eight centers in the study were responsible for all of the recurrences. Either way, more work is needed to address the mesh density question. In addition, the surgeon should remember that a lot of the information on the various meshes comes from animal studies and how these studies correlate with the clinical arena is not known. For example, many animal models show that ePTFE can shrink 25% to 50% when placed on the abdominal wall to mimic hernia repair. This is in sharp contrast to what Schoenmaeckers found in 2009. He had 40 patients who underwent IH repair using ePTFE (DualMesh, WL Gore) and had computed tomography (CT) scans of their abdomen for one reason or another 3 to 59 months later. They had radiologists (blinded to the size of the original implant) measure the transverse diameter of the meshes and found that 28% had no mesh shrinkage and the mean shrinkage of the whole group was only 7.5%.
was 17% versus only 7% for the standard meshes. This may have been due to the technique since 19 of the 20 recurrences had absorbable suture used for mesh fixation and three of the eight centers in the study were responsible for all of the recurrences. Either way, more work is needed to address the mesh density question. In addition, the surgeon should remember that a lot of the information on the various meshes comes from animal studies and how these studies correlate with the clinical arena is not known. For example, many animal models show that ePTFE can shrink 25% to 50% when placed on the abdominal wall to mimic hernia repair. This is in sharp contrast to what Schoenmaeckers found in 2009. He had 40 patients who underwent IH repair using ePTFE (DualMesh, WL Gore) and had computed tomography (CT) scans of their abdomen for one reason or another 3 to 59 months later. They had radiologists (blinded to the size of the original implant) measure the transverse diameter of the meshes and found that 28% had no mesh shrinkage and the mean shrinkage of the whole group was only 7.5%.
Barrier meshes with various antiadhesive coatings to prevent ingrowth to the viscera while promoting ingrowth to the abdominal wall had just become available with the last edition of Mastery of Surgery. Now, 3 years later we have more data and more barriers. The first ones on the scene were the coated polyester mesh Parietex Composite (PCO, Covidien) and the coated PPM Sepramesh (Bard) and the coated PPM Proceed (Ethicon). In 2006 Burger looked at these three meshes along with others placed intraperitoneally in 200 rats. They found that in this model the PCO and Sepramesh had fewest adhesions and maximum incorporation into the abdominal wall and were the meshes of choice for intraperitoneal placement. Pierce in 2009, in a rabbit model, looked at these three barrier meshes (at 120 days) in addition to the newest polypropylene barrier mesh by Atrium called the C-Qur mesh. What is important about this study is that they looked at the tenacity of the adhesions, which is a reflection of ingrowth and is our concern. He found all of the barrier meshes worked as advertised. Proceed was the worst with the highest percent of adhesion tenacity and coverage of the group but all barrier meshes were for the most part effective. This is in contrast to a study from 2009 by Schreinemacher who evaluated the barrier meshes (at 7 and 30 days) placed intraperitoneally in a rat model. He found PCO and C-Qur performed the best at 7 days but at 30 days this effect had diminished and there was an increase in adhesions in all of the meshes. Tenacity of the adhesions was not evaluated. Proceed was again the worst performer at both study points. An interesting finding was that none of the meshes had good incorporation into the abdominal wall except at the sites of suture fixation. It appears these antiadhesive coatings work but “second-look” studies are critical to see what happens in the clinical situation. The first clinical second-look study was with DualMesh, an ePTFE mesh. Koehler along with us and several other investigators had the opportunity to do second-look operations in 65 patients who had had ePTFE placed laparoscopically. We found that 91% had no or filmy adhesions and none had severe or tenacious adhesions. In 2006, Chelala published a series of 400 laparoscopic ventral hernia repairs using PCO and was able to have a second look in 35 of these patients. The vast majority had no adhesions or filmy adhesions. In 2010 he updated his results to a total of 85 second-look operations and found similar results. This is the standard that all barrier coated meshes will have to meet.
In my opinion we do not have enough evidence at this point to recommend one type of synthetic mesh over another. They each have their good and bad points and it is important the surgeon be familiar with these issues. PPM, ePTFE, and polyester all have been used very successfully for many years. While they are not perfect, they play a critical role for the herniologist and no one mesh can be ideal for the many different situations the surgeon encounters.
Biologic Meshes
We now have several more years of experience with these meshes. The common theme with these products is to take human or animal tissue, get rid of the cellular component to avoid allergic reaction, and then stabilize the protein structure so it can act as a scaffold. These products are thus a collagen implant that allow native fibroblasts to deposit more collagen to then make a “biologic” mesh. Many of these meshes are expensive and have been brought to market without long-term evaluation as a permanent solution for hernia repair. Many were evaluated only in the animal lab for use other than hernia repair. Patients with increased risk of poor wound healing or known hernia formers may not remodel biologic meshes with healthy collagen. Unfortunately, the various companies have been promoting these products as permanent solutions and they should not be seen as such until long-term studies have been done. Many more meshes have been introduced from animal sources. Some of these meshes have the collagen cross-linked, which retards the degradation of the donor collagen, and the amount of cross-linking is variable. The more the mesh is cross-linked the longer it remains as part of the final scar created. At present we do not know if cross-linking is good or bad and, if good, how much cross-linking is needed. Some of the meshes require refrigeration, some require rehydration, and some have a ventral and dorsal surface for placement.
We have learned a few things over the past several years. The best use of these products is as a sublay or as an onlay with the mesh closely adherent to host tissues. The biologic meshes require vascular ingrowth to survive and this cannot occur if the graft is used as a bridge. If the surgeon uses these grafts as a bridge between fascial edges then the surgeon and the patient should be prepared for a recurrent hernia. This does not mean that you should never use them as a bridge but that it is not the ideal situation. We also know now that products made from dermis with a lot of elastin will stretch over time and while there may be some scar tissue present it is lax and stretched due to the elastin leftover and the patient appears to have a recurrence. Lastly, the biologic meshes can be used in the face of contamination but not overt infection. They will get infected in the face of gross infection and will disappear quickly as they are degraded by the process. There is a new category of mesh just introduced and is made of bioabsorbable polymers. The only product in this category is the WL Gore product called Bio-A. This acts like a biologic in that it disappears but host cells infiltrate the 3D matrix of the mesh and replace it with vascularized soft tissue. Histology shows the tissue is a mix of type I and type III collagen that matures mainly into type I collagen as the wound matures. We have used a small piece of this to reinforce crural closure in 65 paraesophageal hiatal hernias and found it to work very well and have had no problems in the short term. The company has introduced large sheets of this material for use in IH repair. The main advantage of this material over the biologics is that no refrigeration or rehydration is required and it is much less expensive.
Umbilical and Epigastric Hernias
Omar Askar spent his entire life studying the anterior abdominal wall and I encourage anyone interested in learning the complex anatomy involved to read his work. He
termed these hernias “aponeurotic” hernias to distinguish them from other ventral hernias. The development of an umbilical hernia has to do with the embryology of the abdominal wall. The lower part of the umbilicus has the obliterated vessels and the urachus and is somewhat reinforced and protected from the preperitoneal fat and viscera. However, the upper part is thin transversalis fascia only and pieces of preperitoneal fat and viscera can be extruded forward to initiate hernia formation. Epigastric hernias are hernias of the linea alba. They occur where neurovascular bundles penetrate the fascia and here preperitoneal fat can get into the defect and as intra-abdominal pressure increases for whatever reason the defect enlarges and allows viscera to enter. Both present with a variety of symptoms and regardless of the size, incarceration and strangulation are always a possibility. For this reason, these hernias should be repaired unless there is a strong contraindication. Their size will increase over time and the larger the hernia the more difficult the repair.
termed these hernias “aponeurotic” hernias to distinguish them from other ventral hernias. The development of an umbilical hernia has to do with the embryology of the abdominal wall. The lower part of the umbilicus has the obliterated vessels and the urachus and is somewhat reinforced and protected from the preperitoneal fat and viscera. However, the upper part is thin transversalis fascia only and pieces of preperitoneal fat and viscera can be extruded forward to initiate hernia formation. Epigastric hernias are hernias of the linea alba. They occur where neurovascular bundles penetrate the fascia and here preperitoneal fat can get into the defect and as intra-abdominal pressure increases for whatever reason the defect enlarges and allows viscera to enter. Both present with a variety of symptoms and regardless of the size, incarceration and strangulation are always a possibility. For this reason, these hernias should be repaired unless there is a strong contraindication. Their size will increase over time and the larger the hernia the more difficult the repair.
Repair techniques are numerous and have progressed from the Mayo “vest-over-pants” autogenous repair to the more commonly done mesh-based repairs. As Deysine and others showed, autogenous repair has up to a 40% recurrence rate; thus only the smallest of defects in the small patient should be primarily repaired. Sanjay in 2005 reported on mesh repair versus suture repair in 100 patients with umbilical hernias. Median follow-up was 4.5 years. The recurrence rate for suture repair was 11% and 0% for mesh repair. Arroyo in a 2001 randomized trial found similar results and felt mesh repair should be the standard of care. Schumacher in 2003 found that in patients with a body mass index (BMI) <30 the recurrence rate was 8% while in those with a BMI >30 the recurrence rate was 32%. This was confirmed by Halm recently. Schumacher also found that the larger the defect the higher the chance for recurrence if repaired without mesh. Most patients with umbilical or epigastric hernias are large people or have large hernias and should have mesh repair. As with all ventral hernia repairs, the best results are when the mesh is much larger than the defect and is placed as an onlay or sublay and anchored to good fascia. This is difficult to do with traditional meshes without making a large incision and developing large soft tissue skin and subcutaneous flaps. Until recently the best way to accomplish this was to do the repair laparoscopically since this allows placement of a large piece of mesh behind the defect without making a large incision. This is still the best option in very large hernias in large people. However, there is now an open approach that allows a sublay repair through a small incision.
Bard was the first to introduce a circular patch specifically for umbilical or epigastric hernias. This is a composite mesh made of a PTFE disk on the side that is placed toward the viscera (to prevent ingrowth) in the abdomen and has a smaller disk of PPM on the side that lies against the abdominal wall to aid in ingrowth. There are two straps of PPM that are attached pockets and aid in positioning; the straps are removed during the procedure. In addition there is a positioning ring that helps in placement and allows the patch to lie flat against the peritoneal surface of the abdominal wall. The prosthetic comes in three sizes and has just enough memory to spring open under the fascia and thus one can get a mesh that is larger than the defect and behind it; also, this can be done without making a large incision that would be required for any other prosthesis. Thus, you get the benefits of an underlay prosthesis through a small incision. Ethicon, Atrium and Bard have all introduced a similar product to the PTFE disk. These are all polypropylene-based with antiadhesion barriers to allow intraperitoneal placement. All of these patches have their good and bad points and the surgeon should try all of them to find the one that works best.
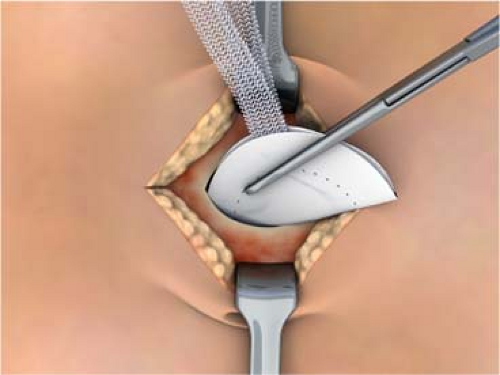
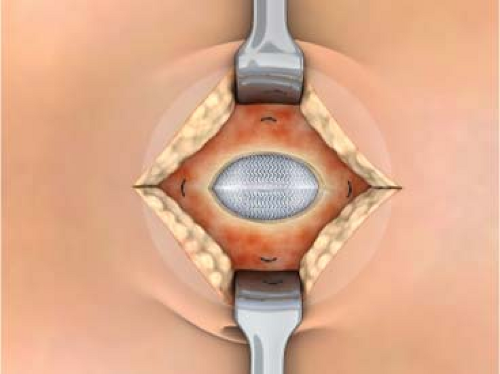
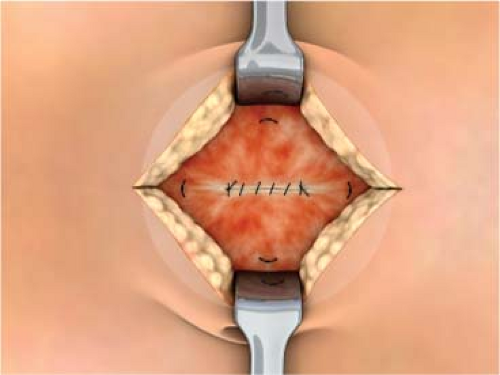
The surgery is begun by making the appropriate skin incision for whatever repair is being done. The hernia sac is entered, the contents reduced, and the sac excised down to fascia level. The peritoneal surface underlying the defect is swept of any adhesions and tissue circumferentially to a diameter equal to the size of the patch selected for use in the repair. There are two different methods at this point based on surgeon’s preference. A forcep or hemostat is used to bring opposite edges together like a taco shell and the prosthesis is placed into the peritoneal cavity. The patch springs open readily due to the placement ring but the surgeon must still look to see if the patch is completely unfolded and is abutting the peritoneal surface 360 degrees without any viscera between the patch and the peritoneal surface. The placement straps can also be used to align the patch properly. Each polypropylene strap is sutured to a fascial edge and the wound closed. We don’t favor this approach since the sutures are not placed into healthy fascia. In addition, the area of the patch where the defect in the fascia is located is not covered by fascia and if there is wound skin breakdown or infection the patch will be exposed. In umbilical hernias the thin umbilical skin is right on the PPM component and this risks erosion. We prefer to dissect the subcutaneous tissues off of the fascia 360 degrees for 2 to 4 cm back to good, healthy fascia (Fig. 1). We then use a 2-0 Prolene double-armed suture to place “U” stitches through the polypropylene component only at the 12, 3, 6, and 9 o’clock positions. We place sutures at the 12 and 6 o’clock positions only for the smallest patch. The sutures are brought up through the fascia 2 to 4 cm back from the edge of the hernia defect through healthy fascia. The sutures are tied and the defect is broadly covered in an underlay fashion but through a small incision (Fig. 2). Since the fat has been dissected off of the fascia, the edges of the fascia are easily closed over the patch, protecting it from any superficial wound problems that may develop. When closing the fascia it is important to not take large
bites since this will distort how the patch lies against the peritoneal surface (Fig. 3).
bites since this will distort how the patch lies against the peritoneal surface (Fig. 3).
The Bard PTFE product is the only one with clinical data of any length at present. Hadi in 2006 looked at 51 patients who had the Ventralex patch placed for umbilical or epigastric hernias. He found one minor wound infection, one seroma, and no recurrences in the short term. In 2008 we published our results with the Ventralex patch in 88 patients undergoing repair of umbilical (68%) and epigastric (30%) hernias. The average BMI was 32 and follow-up ranged from 8 days to 3 years. There were two cases of mesh infections and no recurrences were found. We compared this with a similar number of umbilical hernias done through the laparoscope and presented by us at the American Hernia Society meeting in 2002. The laparoscopic group had no recurrences and no infections but the laparoscopic repair was $1,200 more expensive than the open Ventralex approach. The laparoscopic approach is still the best approach in the morbidly obese patient or the patient with a very large hernia.
Incisional Hernias
IH make up about 80% of the ventral hernias that surgeons encounter. The range for IH rates after laparotomy is from 2% to 11%, which means at least 150,000 patients are going to develop this complication from abdominal surgery. Multiple studies have looked at risk factors for the development of IH. Van’t Riet showed that any type of wound dehiscence led to an IH in 69% of patients at 10 years of follow-up. Hodgson in 2000 performed a meta-analysis and found what Professor Goligher first reported in 1975; closing the fascia at the time of initial laparotomy with a continuous nonabsorbable suture led to the lowest rate of IH. Van’t Riet looked at fascial closure in a similar meta-analysis 2 years later and found that slowly absorbable (i.e., PDS) or nonabsorbable sutures gave similar results but the nonabsorbable sutures caused more long-term pain. Sorensen recently supported what Raymond Read first described many years ago; smoking is a significant independent risk factor (fourfold increase) for development of IH. Raffetto recently reported a ninefold increase in IH formation in patients having abdominal aortic aneurysm surgery via midline laparotomy. Other factors commonly blamed for IH are obesity, malnutrition, steroids, and wound infection.
Rives felt that IH is more than just a hole in the abdominal wall like an umbilical or epigastric hernia and felt it was a “disease” process. Once an IH occurs the natural history is for it to grow. Delay in repair complicates every single aspect of the surgery and leads to increased morbidity so repair should be done as soon as possible. As it grows the chance of complications such as incarceration and strangulation of viscera, atrophy of subcutaneous tissue, thinning of skin, ulceration of the skin, and loss of domain of the viscera occur. The lateral abdominal muscles retract and become fibrotic and this enlarges the defect. This is what Rives called “eventration disease.” Dubay in 2007 in a rat model of chronic IH found that indeed this disease process did occur as the muscle fibers of the abdominal wall atrophied, shortened, and became fibrotic and stiff. All of these things greatly complicate any repair and increase the chance of repair failure, prosthetic infection, and wound problems.
Preoperative preparation of the patient is an important aspect of any IH repair. If the patient is obese, weight loss is very helpful to any subsequent repair and should always be considered and demanded of the patient prior to ventral hernia repair. Weight loss makes the surgery easier and closure much easier and excess skin and fat can be excised, which pleases the patient. We instruct all of our overweight IH patients on an 1,100 to 1,200 cal/day diet with very little fat and sugar. They are assisted with learning about how to increase their activity levels to burn up more calories. As long as the patient is losing weight the surgery is delayed. The patient is instructed on what to look for with incarceration and understand the risks involved. If the patient is very large and cannot lose weight on their own then we suggest a bariatric operation before repairing the IH. Nongastric band operations like the gastric sleeve or bypass are best since the port of the band creates problems when the hernia is repaired.
Lastly, if there is loss of domain of the intestines, preop pneumoperitoneum (PPP) done slowly on an outpatient can be helpful in stretching the abdominal wall prior to surgery to aid in returning the bowel to the abdominal cavity. This was originally described by Moreno in 1947. It is done by choosing a point on the abdominal wall lateral to the semilunar line at the costal margin. The surgeon should start by injecting 500 to 1,000 cc per day or whatever the patient can tolerate. We have our interventional radiology people place the catheter in the abdomen but it can be done by the surgeon. You can increase the amount to 1,500 to 2,000 cc per day and do this over a 10- to 20-day period. You can instill up to 10 to 15 L if needed. Once the hernia sac fills with air it no longer distends and the air goes into the abdominal cavity. Dumont in 2009 measured the change in length of the muscle on CT scan in 18 patients having PPP. He instilled 12 L of air over 14 days and found that the width of the hernia increased by 14 mm, the width of the rectus muscles increased by 15 mm, and the length of the anterolateral muscles increased by 25 to 30 mm. Many clinical studies have reported successful use of PPP with few if any adverse effects.
Lastly, if there is loss of domain of the intestines, preop pneumoperitoneum (PPP) done slowly on an outpatient can be helpful in stretching the abdominal wall prior to surgery to aid in returning the bowel to the abdominal cavity. This was originally described by Moreno in 1947. It is done by choosing a point on the abdominal wall lateral to the semilunar line at the costal margin. The surgeon should start by injecting 500 to 1,000 cc per day or whatever the patient can tolerate. We have our interventional radiology people place the catheter in the abdomen but it can be done by the surgeon. You can increase the amount to 1,500 to 2,000 cc per day and do this over a 10- to 20-day period. You can instill up to 10 to 15 L if needed. Once the hernia sac fills with air it no longer distends and the air goes into the abdominal cavity. Dumont in 2009 measured the change in length of the muscle on CT scan in 18 patients having PPP. He instilled 12 L of air over 14 days and found that the width of the hernia increased by 14 mm, the width of the rectus muscles increased by 15 mm, and the length of the anterolateral muscles increased by 25 to 30 mm. Many clinical studies have reported successful use of PPP with few if any adverse effects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree