I. Mucocutaneous HSV infections
A. Infections in immunosuppressed patients
1. Acute symptomatic first or recurrent episodes: IV acyclovir (5 mg/kg q8h) or oral acyclovir (400 mg qid), famciclovir (500 mg bid or tid), or valacyclovir (500 mg bid) is effective. Treatment duration may vary from 7 to 14 days.
2. Suppression of reactivation disease (genital or oral-labial): IV acyclovir (5 mg/kg q8h) or oral valacyclovir (500 mg bid) or acyclovir (400–800 mg 3–5 times per day) prevents recurrences during the 30-day period immediately after transplantation. Longer-term HSV suppression is often used for persons with continued immunosuppression. In bone marrow and renal transplant recipients, oral valacyclovir (2 g/d) is also effective in reducing cytomegalovirus infection. Oral valacyclovir at a dose of 4 g/d has been associated with thrombotic thrombocytopenic purpura after extended use in HIV-positive persons. In HIV-infected persons, oral acyclovir (400–800 mg bid), valacyclovir (500 mg bid), or famciclovir (500 mg bid) is effective in reducing clinical and subclinical reactivations of HSV-1 and HSV-2.
B. Infections in immunocompetent patients
1. Genital herpes
a. First episodes: Oral acyclovir (200 mg 5 times per day or 400 mg tid), valacyclovir (1 g bid), or famciclovir (250 mg bid) for 7–14 days is effective. IV acyclovir (5 mg/kg q8h for 5 days) is given for severe disease or neurologic complications such as aseptic meningitis.
b. Symptomatic recurrent genital herpes: Short-course (1- to 3-day) regimens are preferred because of low cost, likelihood of adherence, and convenience. Oral acyclovir (800 mg tid for 2 days), valacyclovir (500 mg bid for 3 days), or famciclovir (750 or 1000 mg bid for 1 day, a 1500-mg single dose, or 500 mg stat followed by 250 mg q12h for 3 days) effectively shortens lesion duration. Other options include oral acyclovir (200 mg 5 times per day), valacyclovir (500 mg bid), and famciclovir (125 mg bid for 5 days).
c. Suppression of recurrent genital herpes: Oral acyclovir (400–800 mg bid) or valacyclovir (500 mg daily) is given. Patients with >9 episodes per year should take oral valacyclovir (1 g daily or 500 mg bid) or famciclovir (250 mg bid or 500 mg bid).
2. Oral-labial HSV infections
a. First episode: Oral acyclovir is given (200 mg 5 times per day or 400 mg tid); an oral acyclovir suspension can be used (600 mg/m2 qid). Oral famciclovir (250 mg bid) or valacyclovir (1 g bid) has been used clinically. The duration of therapy is 5–10 days.
b. Recurrent episodes: If initiated at the onset of the prodrome, single-dose or 1-day therapy effectively reduces pain and speeds healing. Regimens include oral famciclovir (a 1500-mg single dose or 750 mg bid for 1 day) or valacyclovir (a 2-g single dose or 2 g bid for 1 day). Self-initiated therapy with 6-times-daily topical penciclovir cream effectively speeds healing of oral-labial HSV. Topical acyclovir cream has also been shown to speed healing.
c. Suppression of reactivation of oral-labial HSV: If started before exposure and continued for the duration of exposure (usually 5–10 days), oral acyclovir (400 mg bid) prevents reactivation of recurrent oral-labial HSV infection associated with severe sun exposure.
3. Surgical prophylaxis of oral or genital HSV infection: Several surgical procedures, such as laser skin resurfacing, trigeminal nerve-root decompression, and lumbar disk surgery, have been associated with HSV reactivation. IV acyclovir (3–5 mg/kg q8h) or oral acyclovir (800 mg bid), valacyclovir (500 mg bid), or famciclovir (250 mg bid) effectively reduces reactivation. Therapy should be initiated 48 h before surgery and continued for 3–7 days.
4. Herpetic whitlow: Oral acyclovir (200 mg) is given 5 times daily (alternative: 400 mg tid) for 7–10 days.
5. HSV proctitis: Oral acyclovir (400 mg 5 times per day) is useful in shortening the course of infection. In immunosuppressed patients or in patients with severe infection, IV acyclovir (5 mg/kg q8h) may be useful.
6. Herpetic eye infections: In acute keratitis, topical trifluorothymidine, vidarabine, idoxuridine, acyclovir, penciclovir, and interferon are all beneficial. Debridement may be required. Topical steroids may worsen disease.
II. Central nervous system HSV infections
A. HSV encephalitis: IV acyclovir (10 mg/kg q8h; 30 mg/kg per day) is given for 10 days or until HSV DNA is no longer detected in cerebrospinal fluid.
B. HSV aseptic meningitis: No studies of systemic antiviral chemotherapy exist. If therapy is to be given, IV acyclovir (15–30 mg/kg per day) should be used.
C. Autonomic radiculopathy: No studies are available. Most authorities recommend a trial of IV acyclovir.
III. Neonatal HSV infections: Oral acyclovir (60 mg/kg per day, divided into 3 doses) is given. The recommended duration of IV treatment is 21 days. Monitoring for relapse should be undertaken. Continued suppression with oral acyclovir suspension should be given for 3–4 months.
IV. Visceral HSV infections
A. HSV esophagitis: IV acyclovir (15 mg/kg per day) is given. In some patients with milder forms of immunosuppression, oral therapy with valacyclovir or famciclovir is effective.
B. HSV pneumonitis: No controlled studies exist. IV acyclovir (15 mg/kg per day) should be considered.
V. Disseminated HSV infections: No controlled studies exist. IV acyclovir (5 mg/kg q8h) should be tried. Adjustments for renal insufficiency may be needed. No definite evidence indicates that therapy will decrease the risk of death.
VI. Erythema multiforme associated with HSV: Anecdotal observations suggest that oral acyclovir (400 mg bid or tid) or valacyclovir (500 mg bid) will suppress erythema multiforme.
VII. Infections due to acyclovir-resistant HSV: IV foscarnet (40 mg/kg IV q8h) should be given until lesions heal. The optimal duration of therapy and the usefulness of its continuation to suppress lesions are unclear. Some patients may benefit from cutaneous application of trifluorothymidine or 5% cidofovir gel.
Increasingly, shorter courses of therapy are being used for recurrent mucocutaneous infection with HSV-1 or HSV-2 in immunocompetent patients. One-day courses of famciclovir and valacyclovir are clinically effective, more convenient, and generally less costly than longer courses of therapy (Table 216-1). These short-course regimens should be reserved for immunocompetent hosts.
SUPPRESSION OF MUCOCUTANEOUS HERPES
Recognition of the high frequency of subclinical reactivation provides a well-accepted rationale for the use of daily antiviral therapy to suppress reactivations of HSV, especially in persons with frequent clinical reactivations (e.g., those with recently acquired genital HSV infection). Immunosuppressed persons, including those with HIV infection, may also benefit from daily antiviral therapy. Recent studies have shown the efficacy of daily acyclovir and valacyclovir in reducing the frequency of HSV reactivations among HIV-positive persons. Regimens used include acyclovir (400–800 mg twice daily), famciclovir (500 mg twice daily), and valacyclovir (500 mg twice daily); valacyclovir at a dose of 4 g/d was associated with thrombotic thrombocytopenic purpura in one study of HIV-infected persons. In addition, daily treatment of HSV-2 reduces the titer of HIV RNA in plasma (0.5-log reduction) and in genital mucosa (0.33-log reduction).
REDUCED HSV TRANSMISSION TO SEXUAL PARTNERS
Once-daily valacyclovir (500 mg) has been shown to reduce transmission of HSV-2 between sexual partners. Transmission rates are higher from males to females and among persons with frequent HSV-2 reactivation. Serologic screening can be used to identify at-risk couples. Daily valacyclovir appears to be more effective at reducing subclinical shedding than daily famciclovir.
ACYCLOVIR RESISTANCE
Acyclovir-resistant strains of HSV have been identified. Most of these strains have an altered substrate specificity for phosphorylating acyclovir. Thus, cross-resistance to famciclovir and valacyclovir is usually found. Occasionally, an isolate with altered TK specificity arises and is sensitive to famciclovir but not to acyclovir. In some patients infected with TK-deficient virus, higher doses of acyclovir are associated with clearing of lesions. In others, clinical disease progresses despite high-dose therapy. Almost all clinically significant acyclovir resistance has been seen in immunocompromised patients, and HSV-2 isolates are more often resistant than HSV-1 strains. A study by the Centers for Disease Control and Prevention indicated that ~5% of HSV-2 isolates from HIV-positive persons exhibit some degree of in vitro resistance to acyclovir. Of HSV-2 isolates from immunocompetent patients attending sexually transmitted disease clinics, <0.5% show reduced in vitro sensitivity to acyclovir. The lack of appreciable change in the frequency of detection of such isolates in the past 20 years probably reflects the reduced transmission of TK-deficient mutants. Isolation of HSV from lesions persisting despite adequate dosages and blood levels of acyclovir should raise the suspicion of acyclovir resistance. Therapy with the antiviral drug foscarnet is useful in acyclovir-resistant cases (Chap. 215e). Because of its toxicity and cost, this drug is usually reserved for patients with extensive mucocutaneous infections. Cidofovir is a nucleotide analogue and exists as a phosphonate or monophosphate form. Most TK-deficient strains of HSV are sensitive to cidofovir. Cidofovir ointment speeds healing of acyclovir-resistant lesions. No well-controlled trials of systemic cidofovir have been reported. True TK-negative variants of HSV appear to have a reduced capacity to spread because of altered neurovirulence—a feature important in the relatively infrequent presence of such strains in immunocompetent populations, even with increasing use of antiviral drugs.
ACYCLOVIR EFFICACY IN THE DEVELOPING WORLD
![]() Early studies of acyclovir-like drugs were performed solely in the developed world. Recent studies have shown that, although acyclovir-like drugs are effective in the developing world, their clinical and virologic benefits seem reduced from those in European and U.S. populations. The mechanism of this phenomenon is uncertain. Acyclovir therapy does not reduce the rate of HIV acquisition; however, HIV load among MSM in the United States decreased by 1.3 log10 in contrast to 0.9 log10 among Peruvian MSM and 0.5 log10 among African women.
Early studies of acyclovir-like drugs were performed solely in the developed world. Recent studies have shown that, although acyclovir-like drugs are effective in the developing world, their clinical and virologic benefits seem reduced from those in European and U.S. populations. The mechanism of this phenomenon is uncertain. Acyclovir therapy does not reduce the rate of HIV acquisition; however, HIV load among MSM in the United States decreased by 1.3 log10 in contrast to 0.9 log10 among Peruvian MSM and 0.5 log10 among African women.
PREVENTION
The success of efforts to control HSV disease on a population basis through suppressive antiviral chemotherapy and/or educational programs will be limited. Barrier forms of contraception (especially condoms) decrease the likelihood of transmission of HSV infection, particularly during periods of asymptomatic viral excretion. When lesions are present, HSV infection may be transmitted by skin-to-skin contact despite the use of a condom. Nevertheless, the available data suggest that consistent condom use is an effective means of reducing the risk of genital HSV-2 transmission. Chronic daily antiviral therapy with valacyclovir can also be partially effective in reducing acquisition of HSV-2, especially among susceptible women. There are no comparative efficacy studies of valacyclovir versus condom use. Most authorities suggest both approaches. The need for a vaccine to prevent acquisition of HSV infection is great, especially in light of the role HSV-2 plays in enhancing the acquisition and transmission of HIV-1.
A substantial portion of neonatal HSV cases could be prevented by reducing the acquisition of HSV by women in the third trimester of pregnancy. Neonatal HSV infection can result from either the acquisition of maternal infection near term or the reactivation of infection at delivery in the already-infected mother. Thus strategies for reducing neonatal HSV are complex. Some authorities have recommended that antiviral therapy with acyclovir or valacyclovir be given to HSV-2-infected women in late pregnancy as a means of reducing reactivation of HSV-2 at term. Data are not available to support the efficacy of this approach. Moreover, the high treatment-to-prevention ratio makes this a dubious public health approach, even though it can reduce the frequency of HSV-associated cesarean delivery.
217 | Varicella-Zoster Virus Infections |
DEFINITION
Varicella-zoster virus (VZV) causes two distinct clinical entities: varicella (chickenpox) and herpes zoster (shingles). Chickenpox, a ubiquitous and extremely contagious infection, is usually a benign illness of childhood characterized by an exanthematous vesicular rash. With reactivation of latent VZV (which is most common after the sixth decade of life), herpes zoster presents as a dermatomal vesicular rash, usually associated with severe pain.
ETIOLOGY
Early in the twentieth century, similarities in the histopathologic features of skin lesions resulting from varicella and herpes zoster were demonstrated. Viral isolates from patients with chickenpox and herpes zoster produced similar alterations in tissue culture—specifically, the appearance of eosinophilic intranuclear inclusions and multinucleated giant cells. These results suggested that the viruses were biologically similar. Restriction endonuclease analyses of viral DNA from a patient with chickenpox who subsequently developed herpes zoster verified the molecular identity of the two viruses responsible for these different clinical presentations.
VZV is a member of the family Herpesviridae, sharing with other members such structural characteristics as a lipid envelope surrounding a nucleocapsid with icosahedral symmetry, a total diameter of ~180–200 nm, and centrally located double-stranded DNA that is ~125,000 bp in length.
PATHOGENESIS AND PATHOLOGY
Primary Infection Transmission occurs readily by the respiratory route; the subsequent localized replication of the virus at an undefined site (presumably the nasopharynx) leads to seeding of the lymphatic/reticuloendothelial system and ultimately to the development of viremia. Viremia in patients with chickenpox is reflected in the diffuse and scattered nature of the skin lesions and can be confirmed in selected cases by the recovery of VZV from the blood or routinely by the detection of viral DNA in either blood or lesions by polymerase chain reaction (PCR). Vesicles involve the corium and dermis, with degenerative changes characterized by ballooning, the presence of multinucleated giant cells, and eosinophilic intranuclear inclusions. Infection may involve localized blood vessels of the skin, resulting in necrosis and epidermal hemorrhage. With the evolution of disease, the vesicular fluid becomes cloudy because of the recruitment of polymorphonuclear leukocytes and the presence of degenerated cells and fibrin. Ultimately, the vesicles either rupture and release their fluid (which includes infectious virus) or are gradually reabsorbed.
Recurrent Infection The mechanism of reactivation of VZV that results in herpes zoster is unknown. Presumably, the virus infects dorsal root ganglia during chickenpox, where it remains latent until reactivated. Histopathologic examination of representative dorsal root ganglia during active herpes zoster demonstrates hemorrhage, edema, and lymphocytic infiltration.
Active replication of VZV in other organs, such as the lung or the brain, can occur during either chickenpox or herpes zoster but is uncommon in the immunocompetent host. Pulmonary involvement is characterized by interstitial pneumonitis, multinucleated giant cell formation, intranuclear inclusions, and pulmonary hemorrhage. Central nervous system (CNS) infection leads to histopathologic evidence of perivascular cuffing similar to that encountered in measles and other viral encephalitides. Focal hemorrhagic necrosis of the brain, characteristic of herpes simplex virus (HSV) encephalitis, develops infrequently in VZV infection.
EPIDEMIOLOGY AND CLINICAL MANIFESTATIONS
Chickenpox Humans are the only known reservoir for VZV. Chickenpox is highly contagious, with an attack rate of at least 90% among susceptible (seronegative) individuals. Persons of both sexes and all races are infected equally. The virus is endemic in the population at large; however, it becomes epidemic among susceptible individuals during seasonal peaks—namely, late winter and early spring in the temperate zone. Much of our knowledge of the disease’s natural history and incidence predates the licensure of the chickenpox vaccine in 1995. Historically, children 5–9 years old are most commonly affected and account for 50% of all cases. Most other cases involve children 1–4 and 10–14 years old. Approximately 10% of the population of the United States over the age of 15 is susceptible to infection. VZV vaccination during the second year of life has dramatically changed the epidemiology of infection, causing a significant decrease in the annualized incidence of chickenpox.
The incubation period of chickenpox ranges from 10 to 21 days but is usually 14–17 days. Secondary attack rates in susceptible siblings within a household are 70–90%. Patients are infectious ~48 h before onset of the vesicular rash, during the period of vesicle formation (which generally lasts 4–5 days), and until all vesicles are crusted.
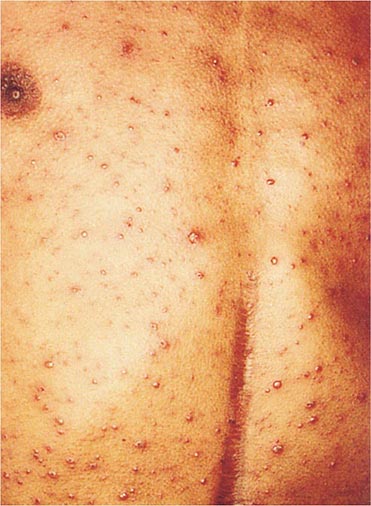
Clinically, chickenpox presents as a rash, low-grade fever, and malaise, although a few patients develop a prodrome 1–2 days before onset of the exanthem. In the immunocompetent patient, chickenpox is usually a benign illness associated with lassitude and with body temperatures of 37.8°–39.4°C (100°–103°F) of 3–5 days’ duration. The skin lesions—the hallmark of the infection—include maculopapules, vesicles, and scabs in various stages of evolution (Fig. 217-1). These lesions, which evolve from maculopapules to vesicles over hours to days, appear on the trunk and face and rapidly spread to involve other areas of the body. Most are small and have an erythematous base with a diameter of 5–10 mm. Successive crops appear over a 2- to 4-day period. Lesions can also be found on the mucosa of the pharynx and/or the vagina. Their severity varies from one person to another. Some individuals have very few lesions, while others have as many as 2000. Younger children tend to have fewer vesicles than older individuals. Secondary and tertiary cases within families are associated with a relatively large number of vesicles. Immunocompromised patients—both children and adults, particularly those with leukemia—have lesions (often with a hemorrhagic base) that are more numerous and take longer to heal than those of immunocompetent patients. Immunocompromised individuals are also at greater risk for visceral complications, which occur in 30–50% of cases and are fatal 15% of the time in the absence of antiviral therapy.
FIGURE 217-1 Varicella lesions at various stages of evolution: vesicles on an erythematous base, umbilical vesicles, and crusts.
The most common infectious complication of varicella is secondary bacterial superinfection of the skin, which is usually caused by Streptococcus pyogenes or Staphylococcus aureus, including strains that are methicillin-resistant. Skin infection results from excoriation of lesions after scratching. Gram’s staining of skin lesions should help clarify the etiology of unusually erythematous and pustulated lesions.
The most common extracutaneous site of involvement in children is the CNS. The syndrome of acute cerebellar ataxia and meningeal inflammation generally appears ~21 days after onset of the rash and rarely develops in the pre-eruptive phase. The cerebrospinal fluid (CSF) contains lymphocytes and elevated levels of protein. CNS involvement is a benign complication of VZV infection in children and generally does not require hospitalization. Aseptic meningitis, encephalitis, transverse myelitis, and Guillain-Barré syndrome can also occur. Reye’s syndrome has been reported in children concomitantly treated with aspirin. Encephalitis is reported in 0.1–0.2% of children with chickenpox. Other than supportive care, no specific therapy (e.g., acyclovir administration) has proved efficacious for patients with CNS involvement.
Varicella pneumonia, the most serious complication following chickenpox, develops more often in adults (up to 20% of cases) than in children and is particularly severe in pregnant women. Pneumonia due to VZV usually has its onset 3–5 days into the illness and is associated with tachypnea, cough, dyspnea, and fever. Cyanosis, pleuritic chest pain, and hemoptysis are frequently noted. Roentgenographic evidence of disease consists of nodular infiltrates and interstitial pneumonitis. Resolution of pneumonitis parallels improvement of the skin rash; however, patients may have persistent fever and compromised pulmonary function for weeks.
Other complications of chickenpox include myocarditis, corneal lesions, nephritis, arthritis, bleeding diatheses, acute glomerulonephritis, and hepatitis. Hepatic involvement, distinct from Reye’s syndrome and usually asymptomatic, is common in chickenpox and is generally characterized by elevated levels of liver enzymes, particularly aspartate and alanine aminotransferases.
Perinatal varicella is associated with mortality rates as high as 30% when maternal disease develops within 5 days before delivery or within 48 h thereafter. Illness in this setting is unusually severe because the newborn does not receive protective transplacental antibodies and has an immature immune system. Congenital varicella, with clinical manifestations of limb hypoplasia, cicatricial skin lesions, and microcephaly at birth, is extremely uncommon.
Herpes Zoster Herpes zoster (shingles) is a sporadic disease that results from reactivation of latent VZV from dorsal root ganglia. Most patients with shingles have no history of recent exposure to other individuals with VZV infection. Herpes zoster occurs at all ages, but its incidence is highest (5–10 cases per 1000 persons) among individuals in the sixth decade of life and beyond. Data suggest that 1.2 million cases occur annually in the United States. Recurrent herpes zoster is exceedingly rare except in immunocompromised hosts, especially those with AIDS.
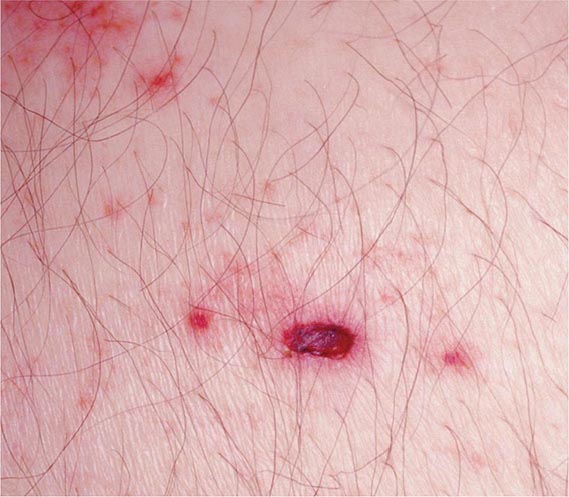
Herpes zoster is characterized by a unilateral vesicular dermatomal eruption, often associated with severe pain. The dermatomes from T3 to L3 are most frequently involved. If the ophthalmic branch of the trigeminal nerve is involved, zoster ophthalmicus results. The factors responsible for the reactivation of VZV are not known. In children, reactivation is usually benign; in adults, it can be debilitating because of pain. The onset of disease is heralded by pain within the dermatome, which may precede lesions by 48–72 h; an erythematous maculopapular rash evolves rapidly into vesicular lesions (Fig. 217-2). In the normal host, these lesions may remain few in number and continue to form for only 3–5 days. The total duration of disease is generally 7–10 days; however, it may take as long as 2–4 weeks for the skin to return to normal. Patients with herpes zoster can transmit infection to seronegative individuals, with consequent chickenpox. In a few patients, characteristic localization of pain to a dermatome with serologic evidence of herpes zoster has been reported in the absence of skin lesions, an entity known as zoster sine herpetica. When branches of the trigeminal nerve are involved, lesions may appear on the face, in the mouth, in the eye, or on the tongue. Zoster ophthalmicus is usually a debilitating condition that can result in blindness in the absence of antiviral therapy. In Ramsay Hunt syndrome, pain and vesicles appear in the external auditory canal, and patients lose their sense of taste in the anterior two-thirds of the tongue while developing ipsilateral facial palsy. The geniculate ganglion of the sensory branch of the facial nerve is involved.
FIGURE 217-2 Close-up of lesions of disseminated zoster. Note lesions at different stages of evolution, including pustules and crusting. (Photo courtesy of Lindsey Baden; with permission.)
In both normal and immunocompromised hosts, the most debilitating complication of herpes zoster is pain associated with acute neuritis and postherpetic neuralgia. Postherpetic neuralgia is uncommon in young individuals; however, at least 50% of zoster patients over age 50 report some degree of pain in the involved dermatome for months after the resolution of cutaneous disease. Changes in sensation in the dermatome, resulting in either hypo- or hyperesthesia, are common.
CNS involvement may follow localized herpes zoster. Many patients without signs of meningeal irritation have CSF pleocytosis and moderately elevated levels of CSF protein. Symptomatic meningoencephalitis is characterized by headache, fever, photophobia, meningitis, and vomiting. A rare manifestation of CNS involvement is granulomatous angiitis with contralateral hemiplegia, which can be diagnosed by cerebral arteriography. Other neurologic manifestations include transverse myelitis with or without motor paralysis.
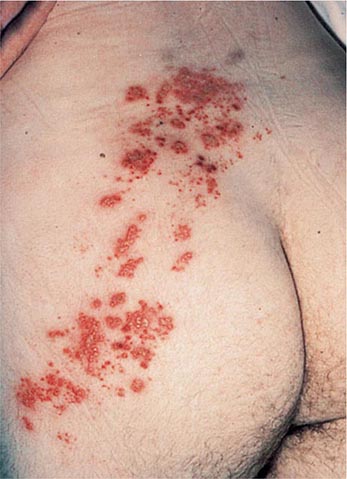
Like chickenpox, herpes zoster is more severe in immunocompromised than immunocompetent individuals. Lesions continue to form for >1 week, and scabbing is not complete in most cases until 3 weeks into the illness. Patients with Hodgkin’s disease and non-Hodgkin’s lymphoma are at greatest risk for progressive herpes zoster. Cutaneous dissemination (Fig. 217-3) develops in ~40% of these patients. Among patients with cutaneous dissemination, the risk of pneumonitis, meningoencephalitis, hepatitis, and other serious complications is increased by 5–10%. However, even in immunocompromised patients, disseminated zoster is rarely fatal.
FIGURE 217-3 Herpes zoster in an HIV-infected patient is seen as hemorrhagic vesicles and pustules on an erythematous base grouped in a dermatomal distribution.
Recipients of hematopoietic stem cell transplants are at particularly high risk of VZV infection. Of all cases of posttransplantation VZV infection, 30% occur within 1 year (50% of these within 9 months); 45% of the patients involved have cutaneous or visceral dissemination. The mortality rate in this situation is 10%. Postherpetic neuralgia, scarring, and bacterial superinfection are especially common in VZV infections occurring within 9 months of transplantation. Among infected patients, concomitant graft-versus-host disease increases the chance of dissemination and/or death.
DIFFERENTIAL DIAGNOSIS
(See also Chap. 25e) The diagnosis of chickenpox is not difficult. The characteristic rash and a history of recent exposure should lead to a prompt diagnosis. Other viral infections that can mimic chickenpox include disseminated HSV infection in patients with atopic dermatitis and the disseminated vesiculopapular lesions sometimes associated with coxsackievirus infection, echovirus infection, or atypical measles. However, these rashes are more commonly morbilliform with a hemorrhagic component rather than vesicular or vesiculopustular. Rickettsialpox (Chap. 211) is sometimes confused with chickenpox; however, rickettsialpox can be distinguished easily by detection of the “herald spot” at the site of the mite bite and the development of a more pronounced headache. Serologic testing is also useful in differentiating rickettsialpox from varicella and can confirm susceptibility in adults unsure of their chickenpox history. Concern about smallpox has recently increased because of the threat of bioterrorism (Chap. 261e). The lesions of smallpox are larger than those of chickenpox and are all at the same stage of evolution at any given time.
Unilateral vesicular lesions in a dermatomal pattern should lead rapidly to the diagnosis of herpes zoster, although the occurrence of shingles without a rash has been reported. Both HSV and coxsackie-virus infections can cause dermatomal vesicular lesions. Supportive diagnostic virology and fluorescent staining of skin scrapings with monoclonal antibodies are helpful in ensuring the proper diagnosis. In the prodromal stage of herpes zoster, the diagnosis can be exceedingly difficult and may be made only after lesions have appeared or by retrospective serologic assessment.
LABORATORY FINDINGS
Unequivocal confirmation of the diagnosis is possible only through the isolation of VZV in susceptible tissue-culture cell lines, the demonstration of either seroconversion or a fourfold or greater rise in antibody titer between acute-phase and convalescent-phase serum specimens, or the detection of VZV DNA by PCR. A rapid impression can be obtained by a Tzanck smear, with scraping of the base of the lesions in an attempt to demonstrate multinucleated giant cells; however, the sensitivity of this method is low (~60%). PCR technology for the detection of viral DNA in vesicular fluid is available in a limited number of diagnostic laboratories. Direct immunofluorescent staining of cells from the lesion base or detection of viral antigens by other assays (such as the immunoperoxidase assay) is also useful, although these tests are not commercially available. The most frequently employed serologic tools for assessing host response are the immunofluorescent detection of antibodies to VZV membrane antigens, the fluorescent antibody to membrane antigen (FAMA) test, immune adherence hemagglutination, and enzyme-linked immunosorbent assay (ELISA). The FAMA test and the ELISA appear to be most sensitive.
PREVENTION
Three methods are used for the prevention of VZV infections. First, a live attenuated varicella vaccine (Oka) is recommended for all children >1 year of age (up to 12 years of age) who have not had chickenpox and for adults known to be seronegative for VZV. Two doses are recommended for all children: the first at 12–15 months of age and the second at ~4–6 years of age. VZV-seronegative persons >13 years of age should receive two doses of vaccine at least 1 month apart. The vaccine is both safe and efficacious. Breakthrough cases are mild and may result in spread of the vaccine virus to susceptible contacts. The universal vaccination of children is resulting in a decreased incidence of chickenpox in sentinel communities. Furthermore, inactivation of the vaccine virus significantly decreases the occurrence of herpes zoster after hematopoietic stem-cell transplantation.
In individuals >50 years of age, a VZV vaccine with 18 times the viral content of the Oka vaccine decreased the incidence of shingles by 51%, the burden of illness by 61%, and the incidence of postherpetic neuralgia by 66%. The Advisory Committee on Immunization Practices has therefore recommended that persons in this age group be offered this vaccine in order to reduce the frequency of shingles and the severity of postherpetic neuralgia.
A second approach is to administer varicella-zoster immune globulin (VZIG) to individuals who are susceptible, are at high risk for developing complications of varicella, and have had a significant exposure. This product should be given within 96 h (preferably within 72 h) of the exposure. Indications for administration of VZIG appear in Table 217-1.
RECOMMENDATIONS FOR VZIG ADMINISTRATION |
Lastly, antiviral therapy can be given as prophylaxis to individuals at high risk who are ineligible for vaccine or who are beyond the 96-h window after direct contact. While the initial studies have used acyclovir, similar benefit can be anticipated with either valacyclovir or famciclovir. Therapy is instituted 7 days after intense exposure. At this time, the host is midway into the incubation period. This approach significantly decreases disease severity, if not totally preventing disease.
218 | Epstein-Barr Virus Infections, Including Infectious Mononucleosis |
DEFINITION
Epstein-Barr virus (EBV) is the cause of heterophile-positive infectious mononucleosis (IM), which is characterized by fever, sore throat, lymphadenopathy, and atypical lymphocytosis. EBV is also associated with several tumors, including nasopharyngeal and gastric carcinoma, Burkitt’s lymphoma, Hodgkin’s disease, and (in patients with immunodeficiencies) B cell lymphoma. The virus is a member of the family Herpesviridae. The two types of EBV that are widely prevalent in nature are not distinguishable by conventional serologic tests.
EPIDEMIOLOGY
![]() EBV infections occur worldwide. These infections are most common in early childhood, with a second peak during late adolescence. By adulthood, more than 90% of individuals have been infected and have antibodies to the virus. IM is usually a disease of young adults. In lower socioeconomic groups and in areas of the world with deficient standards of hygiene (e.g., developing regions), EBV tends to infect children at an early age, and IM is uncommon. In areas with higher standards of hygiene, infection with EBV is often delayed until adulthood, and IM is more prevalent.
EBV infections occur worldwide. These infections are most common in early childhood, with a second peak during late adolescence. By adulthood, more than 90% of individuals have been infected and have antibodies to the virus. IM is usually a disease of young adults. In lower socioeconomic groups and in areas of the world with deficient standards of hygiene (e.g., developing regions), EBV tends to infect children at an early age, and IM is uncommon. In areas with higher standards of hygiene, infection with EBV is often delayed until adulthood, and IM is more prevalent.
EBV is spread by contact with oral secretions. The virus is frequently transmitted from asymptomatic adults to infants and among young adults by transfer of saliva during kissing. Transmission by less intimate contact is rare. EBV has been transmitted by blood transfusion and by bone marrow transplantation. More than 90% of asymptomatic seropositive individuals shed the virus in oropharyngeal secretions. Shedding is increased in immunocompromised patients and those with IM.
PATHOGENESIS
EBV is transmitted by salivary secretions. The virus infects the epithelium of the oropharynx and the salivary glands and is shed from these cells. While B cells may become infected after contact with epithelial cells, studies suggest that lymphocytes in the tonsillar crypts can be infected directly. The virus then spreads through the bloodstream. The proliferation and expansion of EBV-infected B cells along with reactive T cells during IM result in enlargement of lymphoid tissue. Polyclonal activation of B cells leads to the production of antibodies to host-cell and viral proteins. During the acute phase of IM, up to 1 in every 100 B cells in the peripheral blood is infected by EBV; after recovery, 1–50 in every 1 million B cells is infected. During IM, there is an inverted CD4+/CD8+ T cell ratio. The percentage of CD4+ T cells decreases, while there are large clonal expansions of CD8+ T cells; up to 40% of CD8+ T cells are directed against EBV antigens during acute infection. Memory B cells, not epithelial cells, are the reservoir for EBV in the body. When patients are treated with acyclovir, shedding of EBV from the oropharynx stops but the virus persists in B cells.
The EBV receptor (CD21) on the surface of B cells is also the receptor for the C3d component of complement. EBV infection of epithelial cells results in viral replication and production of virions. When B cells are infected by EBV in vitro, they become transformed and can proliferate indefinitely. During latent infection of B cells, only the EBV nuclear antigens (EBNAs), latent membrane proteins (LMPs), and small EBV RNAs (EBERs) are expressed in vitro. EBV-transformed B cells secrete immunoglobulin; only a small fraction of these cells produce virus.
Cellular immunity is more important than humoral immunity in controlling EBV infection. In the initial phase of infection, suppressor T cells, natural killer cells, and nonspecific cytotoxic T cells are important in controlling the proliferation of EBV-infected B cells. Levels of markers of T cell activation and serum interferon γ are elevated. Later in infection, human leukocyte antigen–restricted cytotoxic T cells that recognize EBNAs and LMPs and destroy EBV-infected cells are generated.
If T cell immunity is compromised, EBV-infected B cells may begin to proliferate. When EBV is associated with lymphoma in immunocompetent persons, virus-induced proliferation is but one step in a multistep process of neoplastic transformation. In many EBV-containing tumors, LMP-1 mimics members of the tumor necrosis factor receptor family (e.g., CD40), transmitting growth-proliferating signals.
CLINICAL MANIFESTATIONS
Signs and Symptoms Most EBV infections in infants and young children either are asymptomatic or present as mild pharyngitis with or without tonsillitis. In contrast, ~75% of infections in adolescents present as IM. IM in the elderly often presents with nonspecific symptoms, including prolonged fever, fatigue, myalgia, and malaise. In contrast, pharyngitis, lymphadenopathy, splenomegaly, and atypical lymphocytes are relatively rare in elderly patients.
The incubation period for IM in young adults is ~4–6 weeks. A prodrome of fatigue, malaise, and myalgia may last for 1–2 weeks before the onset of fever, sore throat, and lymphadenopathy. Fever is usually low-grade and is most common in the first 2 weeks of the illness; however, it may persist for >1 month. Common signs and symptoms are listed along with their frequencies in Table 218-1. Lymphadenopathy and pharyngitis are most prominent during the first 2 weeks of the illness, while splenomegaly is more prominent during the second and third weeks. Lymphadenopathy most often affects the posterior cervical nodes but may be generalized. Enlarged lymph nodes are frequently tender and symmetric but are not fixed in place. Pharyngitis, often the most prominent sign, can be accompanied by enlargement of the tonsils with an exudate resembling that of streptococcal pharyngitis. A morbilliform or papular rash, usually on the arms or trunk, develops in ~5% of cases (Fig. 218-1). Many patients treated with ampicillin develop a macular rash; this rash is not predictive of future adverse reactions to penicillins. Erythema nodosum and erythema multiforme also have been described (Chap. 72). The severity of the disease correlates with the levels of CD8+ T cells and EBV DNA in the blood. Most patients have symptoms for 2–4 weeks, but nearly 10% have fatigue that persists for ≥6 months.
SIGNS AND SYMPTOMS OF INFECTIOUS MONONUCLEOSIS |
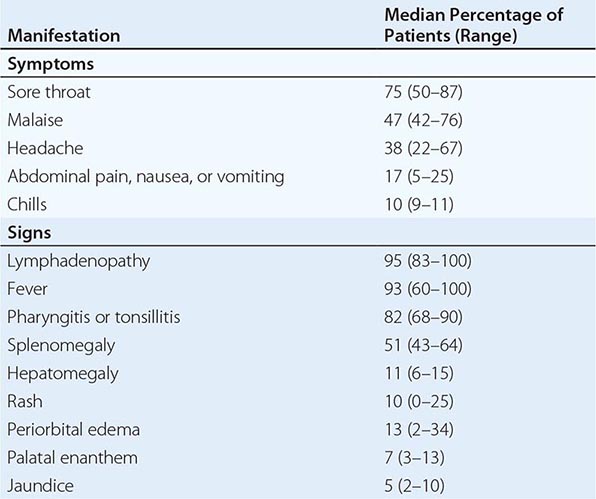
FIGURE 218-1 Rash in a patient with infectious mononucleosis due to Epstein-Barr virus. (Courtesy of Maria Turner, MD; with permission.)
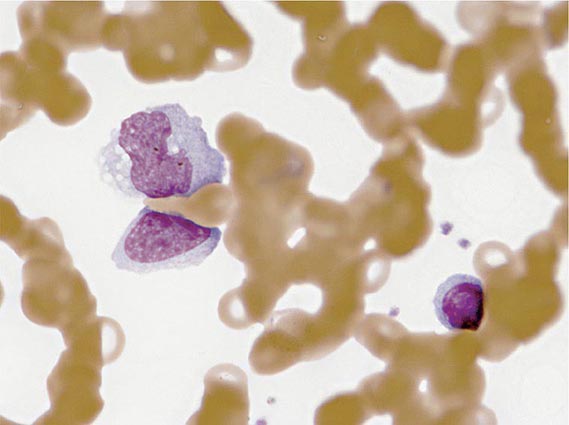
Laboratory Findings The white blood cell count is usually elevated and peaks at 10,000–20,000/μL during the second or third week of illness. Lymphocytosis is usually demonstrable, with >10% atypical lymphocytes. The latter cells are enlarged lymphocytes that have abundant cytoplasm, vacuoles, and indentations of the cell membrane (Fig. 218-2). CD8+ cells predominate among the atypical lymphocytes. Low-grade neutropenia and thrombocytopenia are common during the first month of illness. Liver function is abnormal in >90% of cases. Serum levels of aminotransferases and alkaline phosphatase are usually mildly elevated. The serum concentration of bilirubin is elevated in ~40% of cases.
FIGURE 218-2 Atypical lymphocytes from a patient with infectious mononucleosis due to Epstein-Barr virus.
Complications Most cases of IM are self-limited. Deaths are very rare and are most often due to central nervous system (CNS) complications, splenic rupture, upper airway obstruction, or bacterial superinfection.
When CNS complications develop, they usually do so during the first 2 weeks of EBV infection; in some patients, especially children, they are the only clinical manifestations of IM. Heterophile antibodies and atypical lymphocytes may be absent. Meningitis and encephalitis are the most common neurologic abnormalities, and patients may present with headache, meningismus, or cerebellar ataxia. Acute hemiplegia and psychosis also have been described. The cerebrospinal fluid contains mainly lymphocytes, with occasional atypical lymphocytes. Most cases resolve without neurologic sequelae. Acute EBV infection has also been associated with cranial nerve palsies (especially those involving cranial nerve VII), Guillain-Barré syndrome, acute transverse myelitis, and peripheral neuritis.
Autoimmune hemolytic anemia occurs in ~2% of cases during the first 2 weeks. In most cases, the anemia is Coombs-positive, with cold agglutinins directed against the red blood cell antigen. Most patients with hemolysis have mild anemia that lasts for 1–2 months, but some patients have severe disease with hemoglobinuria and jaundice. Nonspecific antibody responses may also include rheumatoid factor, antinuclear antibodies, anti–smooth muscle antibodies, antiplatelet antibodies, and cryoglobulins. IM has been associated with red-cell aplasia, severe granulocytopenia, thrombocytopenia, pancytopenia, and hemophagocytic lymphohistiocytosis. The spleen ruptures in <0.5% of cases. Splenic rupture is more common among male than female patients and may manifest as abdominal pain, referred shoulder pain, or hemodynamic compromise.
Hypertrophy of lymphoid tissue in the tonsils or adenoids can result in upper airway obstruction, as can inflammation and edema of the epiglottis, pharynx, or uvula. About 10% of patients with IM develop streptococcal pharyngitis after their initial sore throat resolves.
Other rare complications associated with acute EBV infection include hepatitis (which can be fulminant), myocarditis or pericarditis, pneumonia with pleural effusion, interstitial nephritis, genital ulcerations, and vasculitis.
EBV-Associated Diseases Other Than IM EBV-associated lymphoproliferative disease has been described in patients with congenital or acquired immunodeficiency, including those with severe combined immunodeficiency, patients with AIDS, and recipients of bone marrow or organ transplants who are receiving immunosuppressive drugs (especially cyclosporine). Proliferating EBV-infected B cells infiltrate lymph nodes and multiple organs, and patients present with fever and lymphadenopathy or gastrointestinal symptoms. Pathologic studies show B cell hyperplasia or poly- or monoclonal lymphoma.
![]() X-linked lymphoproliferative disease is a recessive disorder of young boys who have a normal response to childhood infections but develop fatal lymphoproliferative disorders after infection with EBV. The protein associated with most cases of this syndrome (SAP) binds to a protein that mediates interactions of B and T cells. Most patients with this syndrome die of acute IM. Others develop hypogammaglobulinemia, malignant B cell lymphomas, aplastic anemia, or agranulocytosis. Disease resembling X-linked lymphoproliferative disease has also been associated with mutations in XIAP. Mutations in ITK, MagT1, or CD27 are associated with inability to control EBV and lymphoma. Moreover, IM has proved fatal to some patients with no obvious preexisting immune abnormality.
X-linked lymphoproliferative disease is a recessive disorder of young boys who have a normal response to childhood infections but develop fatal lymphoproliferative disorders after infection with EBV. The protein associated with most cases of this syndrome (SAP) binds to a protein that mediates interactions of B and T cells. Most patients with this syndrome die of acute IM. Others develop hypogammaglobulinemia, malignant B cell lymphomas, aplastic anemia, or agranulocytosis. Disease resembling X-linked lymphoproliferative disease has also been associated with mutations in XIAP. Mutations in ITK, MagT1, or CD27 are associated with inability to control EBV and lymphoma. Moreover, IM has proved fatal to some patients with no obvious preexisting immune abnormality.
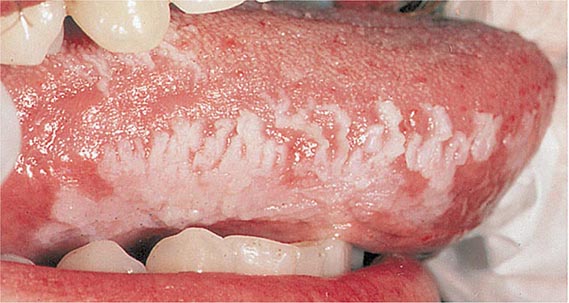
Oral hairy leukoplakia (Fig. 218-3) is an early manifestation of infection with HIV in adults (Chap. 226). Most patients present with raised, white corrugated lesions on the tongue (and occasionally on the buccal mucosa) that contain EBV DNA. Children infected with HIV can develop lymphoid interstitial pneumonitis; EBV DNA is often found in lung tissue from these patients.
FIGURE 218-3 Oral hairy leukoplakia often presents as white plaques on the lateral surface of the tongue and is associated with Epstein-Barr virus infection.
Patients with chronic fatigue syndrome may have titers of antibody to EBV that are elevated but are not significantly different from those in healthy EBV-seropositive adults. While some patients have malaise and fatigue that persist for weeks or months after IM, persistent EBV infection is not a cause of chronic fatigue syndrome. Chronic active EBV infection is very rare and is distinct from chronic fatigue syndrome. The affected patients have an illness lasting >6 months, with elevated levels of EBV DNA in the blood, high titers of antibody to EBV, and evidence of organ involvement, including hepatosplenomegaly, lymphadenopathy, and pneumonitis, uveitis, or neurologic disease.
![]() EBV is associated with several malignancies. About 15% of cases of Burkitt’s lymphoma in the United States and ~90% of those in Africa are associated with EBV (Chap. 134). African patients with Burkitt’s lymphoma have high levels of antibody to EBV, and their tumor tissue usually contains viral DNA. Malaria in African patients may impair cellular immunity to EBV and induce polyclonal B cell activation with an expansion of EBV-infected B cells. These changes may enhance the proliferation of B cells with elevated EBV DNA in the bloodstream, thereby increasing the likelihood of a c-myc translocation—the hallmark of Burkitt’s lymphoma. EBV-containing Burkitt’s lymphoma also occurs in patients with AIDS.
EBV is associated with several malignancies. About 15% of cases of Burkitt’s lymphoma in the United States and ~90% of those in Africa are associated with EBV (Chap. 134). African patients with Burkitt’s lymphoma have high levels of antibody to EBV, and their tumor tissue usually contains viral DNA. Malaria in African patients may impair cellular immunity to EBV and induce polyclonal B cell activation with an expansion of EBV-infected B cells. These changes may enhance the proliferation of B cells with elevated EBV DNA in the bloodstream, thereby increasing the likelihood of a c-myc translocation—the hallmark of Burkitt’s lymphoma. EBV-containing Burkitt’s lymphoma also occurs in patients with AIDS.
Anaplastic nasopharyngeal carcinoma is common in southern China and is uniformly associated with EBV; the affected tissues contain viral DNA and antigens. Patients with nasopharyngeal carcinoma often have elevated titers of antibody to EBV (Chap. 106). High levels of EBV plasma DNA before treatment or detectable levels of EBV DNA after radiation therapy correlate with lower rates of overall survival and relapse-free survival among patients with nasopharyngeal carcinoma.
Worldwide, the most common EBV-associated malignancy is gastric carcinoma. About 9% of these tumors are EBV-positive.
EBV has been associated with Hodgkin’s disease, especially the mixed-cellularity type (Chap. 134). Patients with Hodgkin’s disease often have elevated titers of antibody to EBV. In about half of cases in the United States, viral DNA and antigens are found in Reed-Sternberg cells. The risk of EBV-positive Hodgkin’s disease is significantly increased in young adults for several years after EBV-seropositive IM. About 50% of non-Hodgkin’s lymphomas in patients with AIDS are EBV-positive.
EBV is present in B cells of lesions from patients with lymphomatoid granulomatosis. In some cases, EBV DNA has been detected in tumors from immunocompetent patients with angiocentric nasal NK/T cell lymphoma, T cell lymphoma, and CNS lymphoma. Studies have demonstrated viral DNA in leiomyosarcomas from AIDS patients and in smooth-muscle tumors from organ transplant recipients. Virtually all CNS lymphomas in AIDS patients are associated with EBV. Studies have found that a history of IM and higher levels of antibodies to EBV before the onset of disease is more common in persons with multiple sclerosis than in the general population; additional research on a possible causal relationship is needed.
DIAGNOSIS
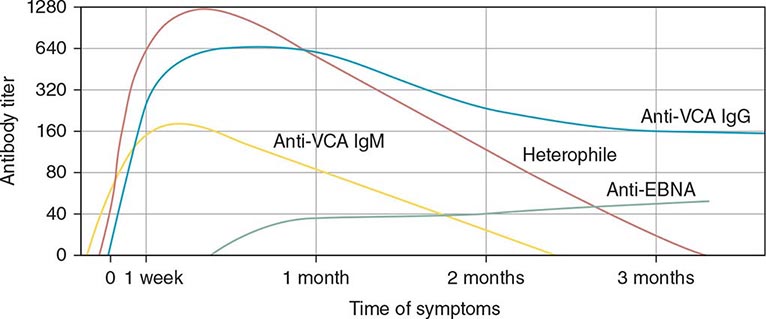
Serologic Testing (Fig. 218-4) The heterophile test is used for the diagnosis of IM in children and adults. In the test for this antibody, human serum is absorbed with guinea pig kidney, and the heterophile titer is defined as the greatest serum dilution that agglutinates sheep, horse, or cow erythrocytes. The heterophile antibody does not interact with EBV proteins. A titer of ≥40 is diagnostic of acute EBV infection in a patient who has symptoms compatible with IM and atypical lymphocytes. Tests for heterophile antibodies are positive in 40% of patients with IM during the first week of illness and in 80–90% during the third week. Therefore, repeated testing may be necessary, especially if the initial test is performed early. Tests usually remain positive for 3 months after the onset of illness, but heterophile antibodies can persist for up to 1 year. These antibodies usually are not detectable in children <5 years of age, in the elderly, or in patients presenting with symptoms not typical of IM. The commercially available monospot test for heterophile antibodies is somewhat more sensitive than the classic heterophile test. The monospot test is ~75% sensitive and ~90% specific compared with EBV-specific serologies (see below). False-positive monospot results are more common among persons with connective tissue disease, lymphoma, viral hepatitis, and malaria.
FIGURE 218-4 Pattern of Epstein-Barr virus (EBV) serology during acute infection. EBNA, Epstein-Barr nuclear antigen; VCA, viral capsid antigen. (From JI Cohen, in NS Young et al [eds]: Clinical Hematology. Philadelphia, Mosby, 2006.)
EBV-specific antibody testing is used for patients with suspected acute EBV infection who lack heterophile antibodies and for patients with atypical infections. Titers of IgM and IgG antibodies to viral capsid antigen (VCA) are elevated in the serum of more than 90% of patients at the onset of disease. IgM antibody to VCA is most useful for the diagnosis of acute IM because it is present at elevated titers only during the first 2–3 months of the disease; in contrast, IgG antibody to VCA is usually not useful for diagnosis of IM but is often used to assess past exposure to EBV because it persists for life. Seroconversion to EBNA positivity also is useful for the diagnosis of acute infection with EBV. Antibodies to EBNA become detectable relatively late (3–6 weeks after the onset of symptoms) in nearly all cases of acute EBV infection and persist for the lifetime of the patient. These antibodies may be lacking in immunodeficient patients and in those with chronic active EBV infection.
Titers of other antibodies also may be elevated in IM; however, these elevations are less useful for diagnosis. Antibodies to early antigens are detectable 3–4 weeks after the onset of symptoms in patients with IM. About 70% of individuals with IM have early antigen diffuse (EA-D) antibodies during the illness; the presence of EA-D antibodies is especially likely in patients with relatively severe disease. These antibodies usually persist for only 3–6 months. Levels of EA-D antibodies are also elevated in patients with nasopharyngeal carcinoma or chronic active EBV infection. Early antigen restricted (EA-R) antibodies are only occasionally detected in patients with IM but are often found at elevated titers in patients with African Burkitt’s lymphoma or chronic active EBV infection. IgA antibodies to EBV antigens have proved useful for the identification of patients with nasopharyngeal carcinoma and of persons at high risk for the disease.
Other Studies Detection of EBV DNA, RNA, or proteins has been valuable in demonstrating the association of the virus with various malignancies. The polymerase chain reaction has been used to detect EBV DNA in the cerebrospinal fluid of some AIDS patients with lymphomas and to monitor the amount of EBV DNA in the blood of patients with lymphoproliferative disease. Detection of high levels of EBV DNA in blood for a few days to several weeks after the onset of IM may be useful if serologic studies yield equivocal results. Culture of EBV from throat washings or blood is not helpful in the diagnosis of acute infection, since EBV persists in the oropharynx and in B cells for the lifetime of the infected individual.
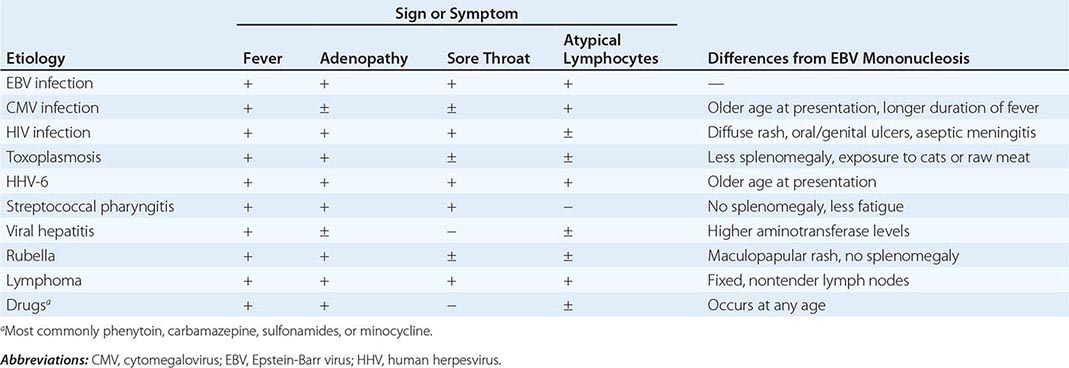
Differential Diagnosis Whereas ~90% of cases of IM are due to EBV, 5–10% of cases are due to cytomegalovirus (CMV) (Chap. 219). CMV is the most common cause of heterophile-negative mononucleosis; less common causes of IM and differences from IM due to EBV are shown in Table 218-2.
DIFFERENTIAL DIAGNOSIS OF INFECTIOUS MONONUCLEOSIS |
PREVENTION
The isolation of patients with IM is unnecessary. A vaccine directed against the major EBV glycoprotein reduced the frequency of IM but did not affect the rate of asymptomatic infection in a phase 2 trial.
219 | Cytomegalovirus and Human Herpesvirus Types 6, 7, and 8 |
CYTOMEGALOVIRUS
DEFINITION
Cytomegalovirus (CMV), which was initially isolated from patients with congenital cytomegalic inclusion disease, is now recognized as an important pathogen in all age groups. In addition to inducing severe birth defects, CMV causes a wide spectrum of disorders in older children and adults, ranging from an asymptomatic subclinical infection to a mononucleosis syndrome in healthy individuals to disseminated disease in immunocompromised patients. Human CMV is one of several related species-specific viruses that cause similar diseases in various animals. All are associated with the production of characteristic enlarged cells—hence the name cytomegalovirus.
CMV, a β-herpesvirus, has double-stranded DNA, four species of mRNA, a protein capsid, and a lipoprotein envelope. Like other herpesviruses, CMV demonstrates icosahedral symmetry, replicates in the cell nucleus, and can cause either a lytic and productive or a latent infection. CMV can be distinguished from other herpesviruses by certain biologic properties, such as host range and type of cytopathology. Viral replication is associated with the production of large intranuclear inclusions and smaller cytoplasmic inclusions. CMV appears to replicate in a variety of cell types in vivo; in tissue culture it grows preferentially in fibroblasts. Although there is little evidence that CMV is oncogenic in vivo, it does transform fibroblasts in rare instances, and genomic transforming fragments have been identified.
EPIDEMIOLOGY
![]() CMV has a worldwide distribution. In many regions of the world, the vast majority of adults are seropositive for CMV, whereas only half of adults in the United States and Canada are seropositive. In regions where the prevalence of CMV antibody is high, immunocompromised adults are more likely to undergo reactivation disease rather than primary infection. Data generated in specific regions should be considered in the context of local seropositivity rates, when appropriate.
CMV has a worldwide distribution. In many regions of the world, the vast majority of adults are seropositive for CMV, whereas only half of adults in the United States and Canada are seropositive. In regions where the prevalence of CMV antibody is high, immunocompromised adults are more likely to undergo reactivation disease rather than primary infection. Data generated in specific regions should be considered in the context of local seropositivity rates, when appropriate.
Of newborns in the United States, ∼1% are infected with CMV; the percentages are higher in many less-developed countries. Communal living and poor personal hygiene facilitate spread. Perinatal and early childhood infections are common. CMV may be present in breast milk, saliva, feces, and urine. Transmission has occurred among young children in day-care centers and has been traced from infected toddler to pregnant mother to developing fetus. When an infected child introduces CMV into a household, 50% of susceptible family members seroconvert within 6 months.
CMV is not readily spread by casual contact but rather requires repeated or prolonged intimate exposure for transmission. In late adolescence and young adulthood, CMV is often transmitted sexually, and asymptomatic carriage in semen or cervical secretions is common. Antibody to CMV is present at detectable levels in a high proportion of sexually active men and women, who may harbor several strains simultaneously. Transfusion of blood products containing viable leukocytes may transmit CMV, with a frequency of 0.14–10% per unit transfused. Transfusion of leukocyte-reduced or CMV-seronegative blood significantly decreases the risk of CMV transmission.
Once infected, an individual generally carries CMV for life. The infection usually remains silent. CMV reactivation syndromes develop more frequently, however, when T lymphocyte–mediated immunity is compromised—for example, after organ transplantation, with lymphoid neoplasms and certain acquired immunodeficiencies (in particular, HIV infection; Chap. 226), or during critical illness in intensive care units. Most primary CMV infections in organ transplant recipients (Chap. 169) result from transmission via the graft. In CMV-seropositive transplant recipients, infection results from reactivation of latent virus or from infection by a new strain. CMV infection may also be associated with diseases as diverse as coronary artery stenosis and malignant gliomas, but these associations require further validation.
PATHOGENESIS
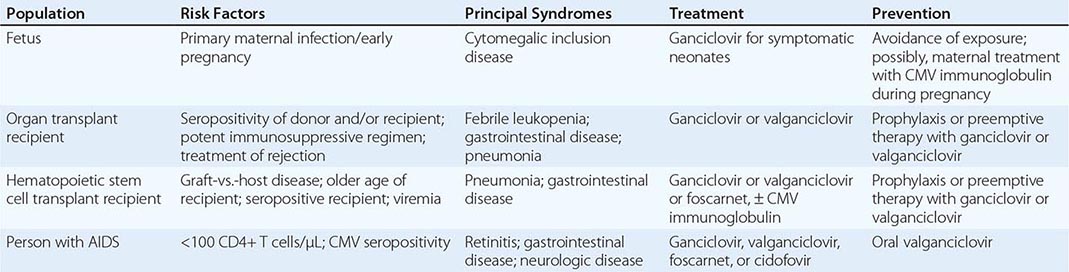
Congenital CMV infection can result from either primary or reactivation infection of the mother. However, clinical disease in the fetus or newborn is related almost exclusively to primary maternal infection (Table 219-1). The factors determining the severity of congenital infection are unknown; a deficient capacity to produce precipitating antibodies and to mount T cell responses to CMV is associated with relatively severe disease.
CMV DISEASE IN THE IMMUNOCOMPROMISED HOST |
Primary infection with CMV in late childhood or adulthood is often associated with a vigorous T lymphocyte response that may contribute to the development of a mononucleosis syndrome similar to that which follows infection with Epstein-Barr virus (Chap. 218). The hallmark of such infection is the appearance of atypical lymphocytes in the peripheral blood; these cells are predominantly activated CD8+ T lymphocytes. Polyclonal activation of B cells by CMV contributes to the development of rheumatoid factors and other autoantibodies during mononucleosis.
Once acquired, CMV persists indefinitely in host tissues. The sites of persistent infection probably include multiple cell types and various organs. Transmission via blood transfusion or organ transplantation is due primarily to latent infections in these tissues. If the host’s T cell responses become compromised by disease or by iatrogenic immunosuppression, latent virus can reactivate to cause a variety of syndromes. Chronic antigenic stimulation in the presence of immunosuppression (for example, after organ transplantation) appears to be an ideal setting for CMV activation and CMV disease. Certain particularly potent suppressants of T cell immunity (e.g., antithymocyte globulin, alemtuzumab) are associated with a high rate of clinical CMV syndromes. CMV may itself contribute to further T lymphocyte hyporesponsiveness, which often precedes superinfection with other opportunistic pathogens such as bacteria, molds, and Pneumocystis.
PATHOLOGY
Cytomegalic cells in vivo (presumed to be infected epithelial cells) are two to four times larger than surrounding cells and often contain an 8- to 10-μm intranuclear inclusion that is eccentrically placed and is surrounded by a clear halo, producing an “owl’s eye” appearance. Smaller granular cytoplasmic inclusions are demonstrated occasionally. Cytomegalic cells are found in a wide variety of organs, including the salivary gland, lung, liver, kidney, intestine, pancreas, adrenal gland, and central nervous system.
The cellular inflammatory response to infection consists of plasma cells, lymphocytes, and monocyte-macrophages. Granulomatous reactions occasionally develop, particularly in the liver. Immunopathologic reactions may contribute to CMV disease. Immune complexes have been detected in infected infants, sometimes in association with CMV-related glomerulopathies. Immune-complex glomerulopathy has also been observed in some CMV-infected patients after renal transplantation.
CLINICAL MANIFESTATIONS
Congenital CMV Infection Fetal infections range from subclinical to severe and disseminated. Cytomegalic inclusion disease develops in ∼5% of infected fetuses and is seen almost exclusively in infants born to mothers who develop primary infections during pregnancy. Petechiae, hepatosplenomegaly, and jaundice are the most common presenting features (60–80% of cases). Microcephaly with or without cerebral calcifications, intrauterine growth retardation, and prematurity are reported in 30–50% of cases. Inguinal hernias and chorioretinitis are less common. Laboratory abnormalities include elevated alanine aminotransferase levels in serum, thrombocytopenia, conjugated hyperbilirubinemia, hemolysis, and elevated protein levels in cerebrospinal fluid. The prognosis for severely infected infants is poor; the mortality rate is 20–30%, and few survivors escape intellectual or hearing difficulties later in childhood. The differential diagnosis of cytomegalic inclusion disease in infants includes syphilis, rubella, toxoplasmosis, infection with herpes simplex virus or enterovirus, and bacterial sepsis.
Most congenital CMV infections are clinically inapparent at birth. Of asymptomatically infected infants, 5–25% develop significant psychomotor, hearing, ocular, or dental abnormalities over the next several years.
Perinatal CMV Infection The newborn may acquire CMV at delivery by passage through an infected birth canal or by postnatal contact with infected breast milk or other maternal secretions. Of infants who are breast-fed for >1 month by seropositive mothers, 40–60% become infected. Iatrogenic transmission can result from blood transfusion; use of leukocyte-reduced or CMV-seronegative blood products for transfusion into low-birth-weight seronegative infants or seronegative pregnant women decreases risk.
The great majority of infants infected at or after delivery remain asymptomatic. However, protracted interstitial pneumonitis has been associated with perinatally acquired CMV infection, particularly in premature infants, and occasionally has been accompanied by infection with Chlamydia trachomatis, Pneumocystis, or Ureaplasma urealyticum. Poor weight gain, adenopathy, rash, hepatitis, anemia, and atypical lymphocytosis may also be found, and CMV excretion often persists for months or years.
CMV Mononucleosis The most common clinical manifestation of CMV infection in immunocompetent hosts beyond the neonatal period is a heterophile antibody–negative mononucleosis syndrome, which may develop spontaneously or follow transfusion of leukocyte-containing blood products. Although the syndrome occurs at all ages, it most often involves sexually active young adults. With incubation periods of 20–60 days, the illness generally lasts for 2–6 weeks. Prolonged high fevers, sometimes with chills, profound fatigue, and malaise, characterize this disorder. Myalgias, headache, and splenomegaly are common, but in CMV (as opposed to Epstein-Barr virus) mononucleosis, exudative pharyngitis and cervical lymphadenopathy are rare. Occasional patients develop rubelliform rashes, often after exposure to ampicillin or certain other antibiotics. Less common are interstitial or segmental pneumonia, myocarditis, pleuritis, arthritis, and encephalitis. In rare cases, Guillain-Barré syndrome complicates CMV mononucleosis. The characteristic laboratory abnormality is relative lymphocytosis in peripheral blood, with >10% atypical lymphocytes. Total leukocyte counts may be low, normal, or markedly elevated. Although significant jaundice is uncommon, serum aminotransferase and alkaline phosphatase levels are often moderately elevated. Heterophile antibodies are absent; however, transient immunologic abnormalities are common and may include the presence of cryoglobulins, rheumatoid factors, cold agglutinins, and antinuclear antibodies. Hemolytic anemia, thrombocytopenia, and granulocytopenia complicate recovery in rare instances.
Most patients recover without sequelae, although postviral asthenia may persist for months. The excretion of CMV in urine, genital secretions, and/or saliva often continues for months or years. Rarely, CMV infection is fatal in immunocompetent hosts; survivors can have recurrent episodes of fever and malaise, sometimes associated with autonomic nervous system dysfunction (e.g., attacks of sweating or flushing).
CMV Infection in the Immunocompromised Host (Table 219-1) CMV is the viral pathogen most commonly complicating organ transplantation (Chap. 169). In recipients of kidney, heart, lung, liver, pancreas, and vascularized composite (hand, face, other) transplants, CMV induces a variety of syndromes, including fever and leukopenia, hepatitis, colitis, pneumonitis, esophagitis, gastritis, and retinitis. CMV disease is an independent risk factor for both graft loss and death. Without prophylaxis, the period of maximal risk is between 1 and 4 months after transplantation. Disease likelihood and viral replication levels generally are greater after primary infection than after reactivation. Molecular studies indicate that seropositive transplant recipients are susceptible to infection with donor-derived, genotypically variant CMV, and such infection often results in disease. Reactivation infection, although common, is less likely than primary infection to be important clinically. The risk of clinical disease is related to various factors, such as degree of immunosuppression, use of antilymphocyte antibodies, lack of anti-CMV prophylaxis, and co-infection with other pathogens. The transplanted organ is particularly vulnerable as a target for CMV infection; thus there is a tendency for CMV hepatitis to follow liver transplantation and for CMV pneumonitis to follow lung transplantation.
CMV viremia occurs in roughly one-third of hematopoietic stem cell transplant recipients; the risk of severe disease may be reduced by prophylaxis or preemptive therapy with antiviral drugs. The risk is greatest 5–13 weeks after transplantation, and identified risk factors include certain types of immunosuppressive therapy, an allogeneic (rather than an autologous) graft, acute graft-versus-host disease, older age, and pretransplantation recipient seropositivity.
CMV is an important pathogen in patients with advanced HIV infection (Chap. 226), in whom it may cause retinitis or disseminated disease, particularly when peripheral-blood CD4+ T cell counts fall below 50–100/μL. As treatment for underlying HIV infection has improved, the incidence of serious CMV infections (e.g., retinitis) has decreased. However, during the first few weeks after institution of highly active antiretroviral therapy, acute flare-ups of CMV retinitis may occur secondary to an immune reconstitution inflammatory syndrome.
Syndromes produced by CMV in immunocompromised hosts often begin with prolonged fatigue, fever, malaise, anorexia, night sweats, and arthralgias or myalgias. Liver function abnormalities, leukopenia, thrombocytopenia, and atypical lymphocytosis may be observed during these episodes. The development of tachypnea, hypoxemia, and unproductive cough signals respiratory involvement. Radiologic examination of the lung often shows bilateral interstitial or reticulonodular infiltrates that begin in the periphery of the lower lobes and spread centrally and superiorly; localized segmental, nodular, or alveolar patterns are less common. The differential diagnosis includes Pneumocystis infection; other viral, bacterial, or fungal infections; pulmonary hemorrhage; and injury secondary to irradiation or to treatment with cytotoxic drugs.
Gastrointestinal CMV involvement may be localized or extensive and almost exclusively affects immunocompromised hosts. Colitis is the most common clinical manifestation in organ transplant recipients. Ulcers of the esophagus, stomach, small intestine, or colon may result in bleeding or perforation. CMV infection may lead to exacerbations of underlying ulcerative colitis. Hepatitis occurs frequently, particularly after liver transplantation. Acalculous cholecystitis and adrenalitis also have been described.
CMV rarely causes meningoencephalitis in otherwise healthy individuals. Two forms of CMV encephalitis are seen in patients with AIDS. One resembles HIV encephalitis and presents as progressive dementia; the other is a ventriculoencephalitis characterized by cranial-nerve deficits, nystagmus, disorientation, lethargy, and ventriculomegaly. In immunocompromised patients, CMV can also cause subacute progressive polyradiculopathy, which is often reversible if recognized and treated promptly.
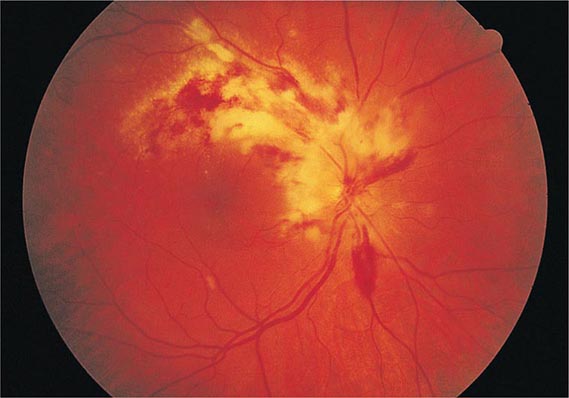
CMV retinitis is an important cause of blindness in immunocompromised patients, particularly patients with advanced AIDS (Chap. 226). Early lesions consist of small, opaque, white areas of granular retinal necrosis that spread in a centrifugal manner and are later accompanied by hemorrhages, vessel sheathing, and retinal edema (Fig. 219-1). CMV retinopathy must be distinguished from that due to other conditions, including toxoplasmosis, candidiasis, and herpes simplex virus infection.
FIGURE 219-1 Cytomegalovirus infection in a patient with AIDS may appear as an arcuate zone of retinitis with hemorrhages and optic disk swelling. Often CMV is confined to the retinal periphery, beyond view of the direct ophthalmoscope.
Fatal CMV infections are often associated with persistent viremia and the involvement of multiple organ systems. Progressive pulmonary infiltrates, pancytopenia, hyperamylasemia, and hypotension are characteristic features that are frequently found in conjunction with a terminal bacterial, fungal, or protozoan superinfection. Extensive adrenal necrosis with CMV inclusions is often documented at autopsy, as is CMV involvement of many other organs.
DIAGNOSIS
CMV infection usually cannot be diagnosed reliably on clinical grounds alone. Isolation of CMV or detection of its antigens or DNA in appropriate clinical specimens is the preferred approach. The most common method of detection is quantitative nucleic acid testing (QNAT) for CMV by polymerase chain reaction (PCR) technology, for which blood or other specimens can be used; some centers use a CMV antigenemia test, an immunofluorescence assay that detects CMV antigens (pp65) in peripheral-blood leukocytes. Such assays may yield a positive result several days earlier than culture methods. QNAT may predict the risk for disease progression, particularly in immunocompromised hosts. CMV DNA in cerebrospinal fluid is useful in the diagnosis of CMV encephalitis or polyradiculopathy. Considerable variation exists among assays and laboratories; a recently introduced international testing standard should help reduce variation in PCR test results.
Virus excretion or viremia is readily detected by culture of appropriate specimens on human fibroblast monolayers. If CMV titers are high, as is common in congenital disseminated infection and in AIDS, characteristic cytopathic effects may be detected within a few days. However, in some situations (e.g., CMV mononucleosis), viral titers are low, and cytopathic effects may take several weeks to appear. Many laboratories expedite diagnosis with an overnight tissue-culture method (shell vial assay) involving centrifugation and an immunocytochemical detection technique employing monoclonal antibodies to an immediate-early CMV antigen. Isolation of virus from urine or saliva does not, by itself, constitute proof of acute infection, since excretion from these sites may continue for months or years after illness. Detection of viremia is a better predictor of acute infection.
A variety of serologic assays detect antibody to CMV. An increased level of IgG antibody to CMV may not be detectable for up to 4 weeks after primary infection. Detection of CMV-specific IgM is sometimes useful in the diagnosis of recent or active infection; however, circulating rheumatoid factors may result in occasional false-positive IgM tests. Serology is especially helpful when used to predict risk of CMV infection and disease in transplant recipients.
PREVENTION
Prevention of CMV in organ and hematopoietic stem cell transplant recipients is usually based on one of two methods: universal prophylaxis or preemptive therapy. With universal prophylaxis, antiviral drugs are used for a defined period, often 3 or 6 months. One clinical trial demonstrated that, in CMV-seronegative recipients with seropositive donors, prophylaxis was more effective at prevention when given for 200 days rather than 100 days. With preemptive therapy, patients are monitored weekly for CMV viremia, and antiviral treatment is initiated once viremia is detected. Because of the bone marrow–suppressive effects of universal prophylaxis, preemptive therapy is more commonly employed in hematopoietic stem cell transplant recipients. For patients with advanced HIV infection (CD4+ T cell counts of <50/μL), some experts have advocated prophylaxis with valganciclovir (see below). However, side effects, lack of proven benefit, possible induction of viral resistance, and high cost have precluded the wide acceptance of this practice. Preemptive therapy is under study in HIV-infected patients.
Several additional measures are useful for the prevention of CMV transmission to CMV-naïve, high-risk patients. The use of CMV-seronegative or leukocyte-depleted blood greatly decreases the rate of transfusion-associated transmission. In a placebo-controlled trial, a CMV glycoprotein B vaccine reduced infection rates among 464 CMV-seronegative women; this outcome raises the possibility that this experimental vaccine will reduce rates of congenital infection, but further studies must validate this approach. A CMV glycoprotein B vaccine with MF59 adjuvant appeared effective in reducing the risk and duration of viremia in both seropositive and seronegative renal transplant recipients at risk for CMV infection. CMV immune globulin has been reported to prevent congenital CMV infection in infants of women with primary infection during pregnancy. Studies in hematopoietic stem cell transplant recipients have produced conflicting results.
Prophylactic acyclovir or valacyclovir may reduce rates of CMV infection and disease in renal transplant recipients, although neither drug is effective in the treatment of active CMV disease.