Chapter 14 Phytochemical variation within a species
As was indicated in Chapter 3, the species represents the unit of plant classification; it may constitute a homogeneous taxon of plants with little variation from one specimen to another or it may include various varieties or races which each have some distinctive feature(s). Thus with Datura stramonium four varieties are described: var. stramonium—white flowers, thorny capsules; var. inermis—white flowers, bald capsules; var. tatula—lilac flowers, thorny capsules and var. godronii—lilac flowers, bald capsules. Often such varieties represent single gene mutations (in the above case two independent genes are involved) and are morphologically recognizable. In other instances the mutation gives rise to a variant having a different secondary metabolite profile—not necessarily discernible in the morphological form; these are termed chemical races or chemodemes. The mutation may involve the presence or absence of a single component or, if acting at an early stage of the biosynthetic route, may involve a whole series of compounds.
Two consequences of the above are, on the positive side, it provides the possibility for the selection and establishment of superior strains with respect to the chemical constituents as described in this chapter. The negative aspect concerns the arrival on the drug market of material which is low in active constituents. This, together with other factors, necessitates the need for strict quality control (Chapter 16) which is being vigorously addressed by various legislative bodies (Chapter 2). Transgenic crops present their own problems.
CHEMICAL RACES, CHEMOTYPES, CHEMODEMES
Before the existence of a chemical race can be established, certain fundamental observations are necessary. A chemical analysis of a number of random samples of a particular species may show a variation between the samples but would be insufficient to demonstrate any genetical differences, since factors such as age, climate and soil can all exert profound effects on the result of the ultimate analysis. Samples of seed, or clones from different plants, must be raised together under uniform conditions, and to exclude hybrids, which do not breed true, cultivation for a number of generations is desirable. It may then be possible to demonstrate that differences occur in either the nature or quantity of a particular constituent and that these differences are of a hereditary nature.
Anthraquinones
The purgative anthraquinone drugs owe their activity to complex mixtures of the 1,8-dihydroxy derivatives of anthranols, their glycosides and free anthraquinones. The relative proportions of the constituents of the mixture, which greatly influence the pharmacological activity, depend not only on time of collection, age of plant, drying conditions and geographical source, but also on genetical factors. In a programme involving Rheum palmatum, van Os produced races varying in their rhein/chrysophanol ratio and other hereditary strains for high- and low-yielding total anthraquinones. The analysis of individual Cassia angustifolia plants has indicated that selection of individuals for high sennoside B-yielding strains is a possibility.
Cardiac glycosides
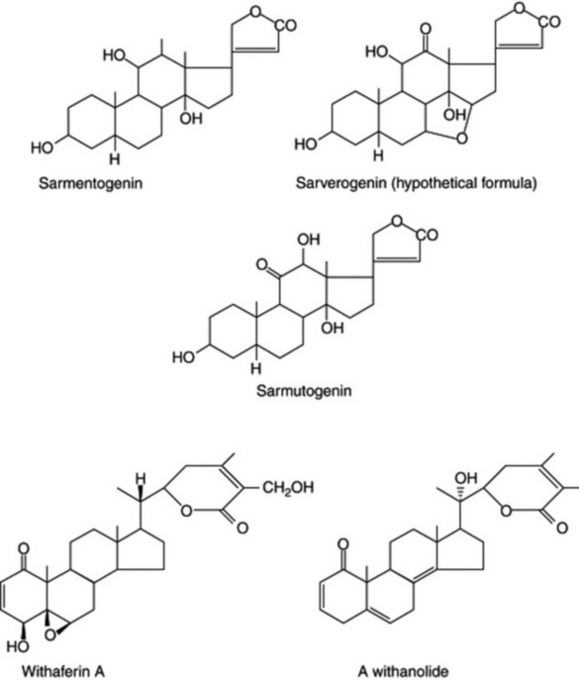
Following intensive chemical investigation of the genus Strophanthus, Reichstein and his colleagues differentiated four chemical varieties of the polymorphous S. sarmentosus from different geographical sources. They are sarverogenin-, sarmentogenin- and sarmutogenin-producing types with glycosides of these, and a fourth form which has a low glycosidal content (Fig. 14.1). Although the locality of growth may produce quantitative differences in the constituents of the various races, the overall type is genetically controlled. Similar variation may exist among those plants that yield steroidal saponins, several thousand of which, from different localities have been screened for their sapogenin content.
Withanolides
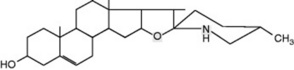
The plant Withania somnifera (Solanaceae), in addition to producing alkaloids, contains steroidal lactones. Investigations over the years, carried out on various sources of plant material, and concerning the non-alkaloidal constituents, had given differing results, which were explained by the work of Abraham et al. (1968) on Israeli plants. Three chemotypes were discovered among 24 populations of W. somnifera collected in various parts of the country. Chemotype I contained predominantly withaferin A (0.2% of the dry weight), which is the principle responsible for the plant’s bacteriostatic and antitumor properties. Chemotype II contains a compound of similar structure, and chemotype III a mixture of related compounds comprising a group of steroidal lactones—the withanolides (Fig. 14.1). The only morphological difference observed between the chemotypes was a difference in flowering time (12 days early) for chemotype III. Since then, other chemotypes of W. somnifera have been reported from India and South Africa.
Steroidal alkaloids
Solanum spp. (Solanaceae) contain steroidal glycosidic alkaloids some of which have been investigated as potential intermediates in corticosteroid synthesis. In S. dulcamara (the woody nightshade) Sander has distinguished a west European tomatidenol group and an east European soladulcidine-solasodine group (Table 14.1). Although polyploid forms do occur in the genus, these chemical varieties all had 2n = 24 chromosomes and were genetically stable. Subsequent work demonstrated that the different chemotypes can occur in the same locality. With the commercial species, about 3500 individual 6-month-old Solanum laciniatum and S. aviculare were analysed by radioimmunoassay (q.v.) and found to contain average leaf concentrations of 1.6–1.7% solasodine; from these a few individuals were selected for future breeding work.
Table 14.1 Chemical races of Solanum dulcamara.
| Aglycone | Sugars | Glycoside |
|---|---|---|
| Soladulicidine (25D) | Galatose (1 mol) | Soladulcidine-tetraoside |
| Glucose (2 mol) | ||
| Xylose (1 mol) | ||
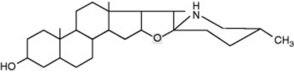 | ||
| Solasodine (25D) | Galactose (1 mol) | |
| Glucose (1 mol) | Solasonine | |
| Rhamnose (1 mol) | ||
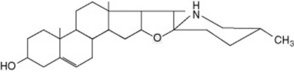 | ||
| Δ5-Tomatidenol (25L) | Galactose (1 mol) | |
| Glucose (1 mol) | α-Solamarine | |
| Rhamnose (1 mol) | ||
 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree