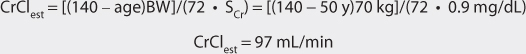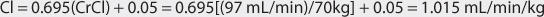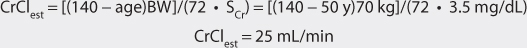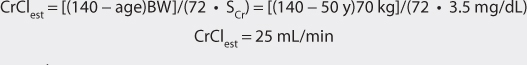FIGURE 5-1 Concentration/time plot for vancomycin 1000 mg given as a 1-hour infusion (circles with dashed line). When given as a 1-hour infusion, end-of-infusion concentrations are higher because the serum and tissues are not in equilibrium. A ½- to 1-hour waiting time for vancomycin distribution to tissues is allowed before peak concentrations are measured.
Trough concentrations (predose or minimum concentrations usually obtained within 30 minutes of the next dose) are usually related to therapeutic outcome for vancomycin because the antibiotic follows time-dependent bacterial killing.1 Optimal bactericidal effects are found at concentrations three to five times the organism’s MIC.1,2 Because the average vancomycin MICs for Staphylococcus aureus and Staphylococcus epidermidis are 1-2 μg/mL, from a historic perspective minimum predose or trough steady-state concentrations equal to 5-10 μg/mL were usually adequate to resolve infections with susceptible organisms through the year 2000. Development of methicillin-resistant Staphylococcus aureus (MRSA) during the early 2000s with MICs of 1.5-2 μg/mL required higher steady-state trough concentrations to achieve a clinical cure.9–12 The need for higher trough concentrations in institutions with antibiograms that included MRSA with higher MIC values lead to the expansion of the therapeutic trough concentration range to 5-15 μg/mL.
Vancomycin penetrates into lung tissue poorly (average serum:tissue ratio of 6:1, average epithelial lining fluid:plasma concentrations ratio of 0.68) and pulmonary concentrations are highly variable among patients.13–15 Based on these findings and reports of therapeutic failures, current treatment guidelines recommend vancomycin steady-state trough concentrations equal to 10-15 μg/mL for lower-intensity dosing and 15-20 μg/mL for complicated infections due to MRSA, such as bacteremia, endocarditis, meningitis, osteomyelitis, severe skin infections, and hospital-acquired pneumonia.16–18 Steady-state vancomycin trough levels less than 10 μg/mL are discouraged due to the possibility of lower levels contributing to treatment failure or to the development of resistance. The development of vancomycin intermediate-level–resistant S. aureus (VISA) and heterogeneously resistant isolates of MRSA (heterogeneous vancomycin-intermediate S. aureus [hVISA]), a mixed colony of sensitive and resistant MRSA subpopulations] during therapy with vancomycin appears to be an important factor in treatment failures.9,19–21
Beginning in the mid-1990s, infectious disease specialists began to recognize the importance of pharmacodynamic principles when treating infections.22–25 Measures such as Cmax/MIC for concentration-dependent antibiotics (where Cmax is the maximum antibiotic concentration), t > MIC for time-dependent antibiotics (time when antibiotic concentrations are above MIC), and AUC/MIC (where AUC is the area under the concentration-time curve for the antibiotic) were recognized as parameters that could be used to help define the response to antibiotic treatment as a combination of exposure to the antibiotic (AUC) and bacterial sensitivity (MIC).
For the treatment of S. aureus pneumonia, this concept was put to the test with vancomycin. In two retrospective studies, it was found that the AUC24/MIC > 400 corresponded to a statistically higher clinical cure rate for patients with MRSA or MSSA (methicillin-sensitive S. aureus).26,27 AUC24 is the area under the concentration-time curve for 24 hours of antibiotic treatment. Thus, if the antibiotic is administered every 8 hours, AUC24 includes three dosage intervals, but if the antibiotic is administered every 24 hours, AUC24 includes only one dosage interval. For these investigations, AUC24 was not computed using patient vancomycin serum concentrations. Instead, it was calculated by taking the quotient of the daily vancomycin dose and an approximation of vancomycin clearance determined from an estimation of creatinine clearance (CrClest) using the Cockcroft-Gault equation (AUC24 = D/{[(CrClest • 0.79) + 15.4] • 0.06}, where D is the daily vancomycin dose).28,29 However, computed AUC24 was validated in one of the later investigations by determining AUC24 using a Bayesian pharmacokinetic computer program in 30 patients and comparing the measured and estimated values. MIC was measured using a broth microdilution method. In the earlier trial, 70 pneumonia patients were studied (50% MSSA isolates, 50% MRSA isolates) with clinical success in 78% for AUC24/MIC >345). In the later trial, 90 pneumonia patients were studied (57 MSSA infections, 33 MRSA infections) with clinical success in 78% for AUC24/MIC >400). Among the patient risk factors for treatment failure were intensive care patients, ventilator support, low serum albumin, MRSA isolate as infecting organism, multilobe lung involvement, high baseline APACHE II (Acute Physiology and Chronic Health Evaluation, second edition) score.
Since the advent of these investigations, AUC24/MIC > 400 has become an accepted standard for the treatment of all S. aureus infections.16,17 Some studies have assessed this threshold for MRSA bacteremia and MRSA endocarditis, while others have derived their own AUC24/MIC ratios using a variety of techniques to estimate or measure AUC24. Either broth microdilution or Etest® has been used to determine MIC.30–36 Obviously, different methodologies to determine AUC24 or MIC can influence the AUC24/MIC value. Other studies are available that use simulations to study the impact of AUC24/MIC > 400 for vancomycin on the treatment of staphylococcal infections.37–44 Also obvious is the fact that the results of studies involving only simulations must be judged differently than retrospective or prospective clinical trials.
Of course, there are other important patient and bacterial factors (such as site of infection, intact host immunologic response, other concurrent patient disease states, bacteria-specific genetic and virulence differences, magnitude of bacterial load invoking an inoculum effect, etc) that need to be considered when assessing antibiotic response. Hopefully, future pharmacokinetic/pharmacodynamic (PK/PD) investigations with vancomycin will include prospective clinical trials for individual infection sites and specific pathogens where AUC24 is measured using drug serum concentrations and MIC values are measured using uniform laboratory methods. This critical information is needed in order to seriously contemplate using this approach in the clinical setting. In lieu of this data, there is some information that indicates achieving steady-state vancomycin trough concentrations of 15-20 μg/mL for serious invasive MRSA infections is effective for bacteria with a MIC 2 μg/mL or less.11,12,45
Vancomycin-associated ototoxicity is usually first noted by the appearance of tinnitus, dizziness, or high-frequency hearing loss (>4000 Hz).4,7,46 Because the hearing loss is initially at high frequencies, the auditory deficit can be challenging to detect unless audiometry is conducted at baseline before drug is administered and during vancomycin treatment. Because audiometry is difficult to conduct in seriously ill patients, it is rarely done in patients receiving ototoxic drugs so clinicians should monitor for signs and symptoms that may indicate that ototoxicity is occurring in a patient (auditory: tinnitus, feeling of fullness or pressure in the ears, loss of hearing acuity in the conversational range; vestibular: loss of equilibrium, headache, nausea, vomiting, vertigo, dizziness, nystagmus, ataxia). Ototoxicity can be permanent if appropriate changes in vancomycin dosing are not made.4,7,46,47 Steady-state trough vancomycin concentrations in excess of 20 μg/mL are associated with ototoxicity, and other patient risk factors include age more than 53 years and preexisting hearing loss. For this patient subgroup, the ototoxicity rate is 19%. While vancomycin ototoxicity usually occurs bilaterally, unilateral changes have been noted.48 In some reports of vancomycin-induced ototoxicity, it is unclear when vancomycin serum concentrations were obtained during the dosage interval so the exact association between concentrations and ototoxicity is uncertain.
Trough vancomycin steady-state concentrations of more than 15 μg/mL are related to an increased incidence of nephrotoxicity, with reported rates as high as 30%-35%.12,49–55 Coadministration of an aminoglycoside with vancomycin may double the risk of developing renal toxicity. Many patients receiving vancomycin are critically ill, so other sources of renal dysfunction, such as dehydration, hypotension, or other nephrotoxic drug therapy (such as aminoglycosides, amphotericin B, loop diuretics, or immunosuppressants) should be ruled out before the diagnosis of vancomycin-induced renal damage is made in a patient. Compared to aminoglycoside antibiotics, vancomycin is usually considered to have less nephrotoxicity potential, but this impression was formed when target steady-state trough levels for vancomycin were in the 5 to 10 μg/mL range.56 In contrast to ototoxicity, vancomycin-related nephrotoxicity is usually reversible with a low incidence of residual damage if the antibiotic is withdrawn or doses appropriately adjusted soon after renal function tests change. With adequate patient monitoring and dosage adjustments, the only result of vancomycin nephrotoxicity may be transient serum creatinine increases of 0.5-2.0 mg/dL.52,57 However, if kidney damage progresses to renal failure, the cost of maintaining the patient on dialysis until kidney function returns can exceed $50,000-100,000 and, if the patient is critically ill, may contribute to his or her death.
Nephrotoxicity and ototoxicity cannot be completely avoided when using vancomycin by keeping serum concentrations within the suggested ranges. However, by adjusting vancomycin dosage regimens so that potentially toxic serum concentrations are avoided, drug concentration–related adverse effects should be held to the absolute minimum.
CLINICAL MONITORING PARAMETERS
Clinicians should always consult the patient’s chart to confirm that antibiotic therapy is appropriate for current microbiologic cultures and sensitivities. Antibiograms should be consulted regularly to note changes in resistance patterns and minimum inhibitory concentrations for pathogens. Also, it should be confirmed that the patient is receiving other appropriate concurrent antibiotic therapy, such as aminoglycosides, when necessary to treat the infection. Patients with severe infections usually have elevated white blood cell counts and body temperatures. Measurement of serial white blood cell counts and body temperatures is useful to determine the efficacy of antibiotic therapy. A white blood cell count with a differential will identify the types of white blood cells that are elevated. A large number of neutrophils and immature neutrophils, clinically known as a “shift to the left,” can also be observed in patients with severe bacterial infections. Favorable response to antibiotic treatment is usually indicated by high white blood cell counts decreasing toward the normal range, the trend of body temperatures (plotted as body temperature vs time, also known as the “fever curve”) approaching normal, and any specific infection site tests or procedures resolving. For instance, in pneumonia patients the chest x-ray should be resolving, in patients with infective endocarditis the size of the bacterial vegetation on the heart valve should be decreasing, or in patients with a wound infection the wound should be less inflamed with less purulent discharge. Clinicians should also be aware that immunocompromised patients with a bacterial infection may not be able to mount a fever or elevated white blood cell count.
Vancomycin steady-state serum concentrations should be measured in 3-5 estimated half-lives. Methods to estimate this parameter are given in the initial dose calculation portion of this chapter. Because prolongation of the dosage interval is often used in patients with decreased elimination, a useful clinical rule is to measure serum concentrations after the third dose. If this approach is used, the dosage interval is increased in tandem with the increase in half-life so that 3-5 half-lives have elapsed by the time the third dose is administered. Additionally, the third dose typically occurs 1-3 days after dosing has commenced and this is a good time to also assess clinical efficacy of the treatment. Steady-state serum concentrations, in conjunction with clinical response, are used to adjust the antibiotic dose, if necessary. Methods for adjusting vancomycin doses using serum concentrations are discussed later in this chapter. If the dosage is adjusted, vancomycin elimination changes or laboratory and clinical monitoring indicate that the infection is not resolving or worsening, or the patient exhibits a potential adverse drug reaction, clinicians should consider rechecking steady-state drug concentrations.
While some clinicians continue to monitor both steady-state peak and trough vancomycin serum concentrations, most individuals advocate the measurement of just a steady-state trough concentration.11,12,18,58,59 The reasoning behind this approach is that vancomycin follows time-dependent bacterial killing, and the efficacy of the drug should be most closely related to the minimum serum concentration encountered over the dosage interval. Current treatment guidelines recommend vancomycin steady-state trough concentrations equal to 10-15 μg/mL for lower-intensity dosing and 15-20 μg/mL for complicated infections due to MRSA, such as bacteremia, endocarditis, meningitis, osteomyelitis, severe skin infections, and hospital-acquired pneumonia.16–18 Steady-state vancomycin trough levels of less than 10 μg/mL are discouraged due to the possibility of lower levels contributing to treatment failure or to the development of resistance. There is some information that indicates achieving steady-state vancomycin trough concentrations of 15-20 μg/mL for serious invasive MRSA infections is effective for bacteria with a MIC of 2 μg/mL or less.11,12,45 Because nephrotoxicity and ototoxicity are related to high trough concentrations, measurement of this value should ensure therapeutic, nontoxic drug concentrations as much as possible.
Vancomycin has a moderate-sized volume of distribution (∼0.7 L/kg) and does not significantly change for most disease states or conditions. Based on this, the argument has been made that if a patient has a therapeutic steady-state trough concentration (15-20 μg/mL) and the dose is in the usual range (1000-2000 mg), it is difficult to produce a steady-state peak concentration that would be above the accepted toxic range (>80 μg/mL).60 While these arguments are intellectually sound and appealing, one of the reasons to measure drug serum concentrations is pharmacokinetic variability. If a patient developed toxicity while receiving vancomycin, it could be difficult to prove that steady-state peak concentrations were in the acceptable range if no serum concentrations were obtained at that time. Clinicians should consider measuring peak concentrations for vancomycin when large doses are given (>1500 mg/dose), for infections that require high peak concentrations (such as central nervous system infections), or if the determination of a peak concentration could aid in the pharmacokinetic computation of the best dose.
Serial monitoring of serum creatinine concentrations should be used to detect nephrotoxicity. Ideally, a baseline serum creatinine concentration is obtained before vancomycin therapy is initiated and three times weekly during treatment. An increasing serum creatinine test on two or more consecutive measurement occasions indicates that more intensive monitoring of serum creatinine values, such as daily, is needed. If serum creatinine measurements increase more than 0.5 mg/dL over the baseline value (or >25%-30% over baseline for serum creatinine values >2 mg/dL) and other causes of declining renal function have been ruled out (other nephrotoxic drugs or agents, hypotension, etc), alternatives to vancomycin therapy or, if that option is not possible, intensive vancomycin serum concentration monitoring should be initiated to ensure that excessive amounts of vancomycin do not accumulate in the patient.
In the clinical setting, audiometry is rarely used to detect ototoxicity because it is difficult to accomplish in severely ill patients. Instead, clinical signs and symptoms of auditory (decreased hearing acuity in the conversational range, feeling of fullness or pressure in the ears, tinnitus) or vestibular (loss of equilibrium, headache, nausea, vomiting, vertigo, nystagmus, ataxia) ototoxicity are monitored at the same time intervals as serum creatinine determination. When high vancomycin concentrations are needed for therapeutic reasons (trough >20 μg/mL, peak >50-60 μg/mL), assessment of renal function and auditory/vestibular function should be conducted on a daily basis. Vancomycin can also cause allergic symptoms such as chills, fever, skin rashes, and anaphylactoid reactions.
BASIC CLINICAL PHARMACOKINETIC PARAMETERS
Vancomycin is almost completely eliminated unchanged in the urine primarily by glomerular filtration (≥90%; Table 5-1).61 This antibiotic is given by short-term (1 hour) intermittent intravenous infusion. Intramuscular administration is usually avoided because this route has been reported to cause tissue necrosis at the site of injection. Oral bioavailability is poor (<10%) so systemic infections cannot be treated by this route of administration.5 However, patients with renal failure who have been given oral vancomycin for the treatment of antibiotic-associated colitis have accumulated therapeutic concentrations because gut wall inflammation increased vancomycin bioavailability and renal dysfunction decreased drug clearance.62–65 Plasma protein binding is ∼55%.66
The manufacturer recommended dose for vancomycin in patients with normal renal function is 30 mg/kg/d given as two or four divided daily doses. In normal-weight adults, the dose is usually 2 g/d given as 1000 mg every 12 hours. However, these doses will not usually achieve recommended trough concentrations to treat serious infections for patients with normal renal function. Current guidelines recommend an initial maintenance dose of 15-20 mg/kg every 8-12 hours for patients with normal renal function, followed by dosage adjustment guided by steady-state vancomycin trough concentrations obtained just before the fourth dose. For seriously ill patients, a loading dose of 25-30 mg/kg can be administered.17 Loading doses can be particularly useful to rapidly attain effective vancomycin concentrations for patients with poor renal function.
Because vancomycin follows time-dependent pharmacodynamics, some clinicians advocate administering vancomycin as a continuous intravenous infusion, usually preceded by an intravenous loading dose. In these cases, the steady-state vancomycin concentration is titrated by changing the infusion rate of the drug.67–72
EFFECTS OF DISEASE STATES AND CONDITIONS ON VANCOMYCIN PHARMACOKINETICS AND DOSING
Nonobese adults with normal renal function (creatinine clearance >80 mL/min, Table 5-1) have an average vancomycin half-life of 8 hours (range = 7-9 hours), and the average volume of distribution for vancomycin is 0.7 L/kg (range = 0.5-1.0 L/kg) in this population.73,74 Because of the moderate size for volume of distribution, fluid balance (under- or overhydration) is less of an issue with vancomycin compared to the aminoglycoside antibiotics.
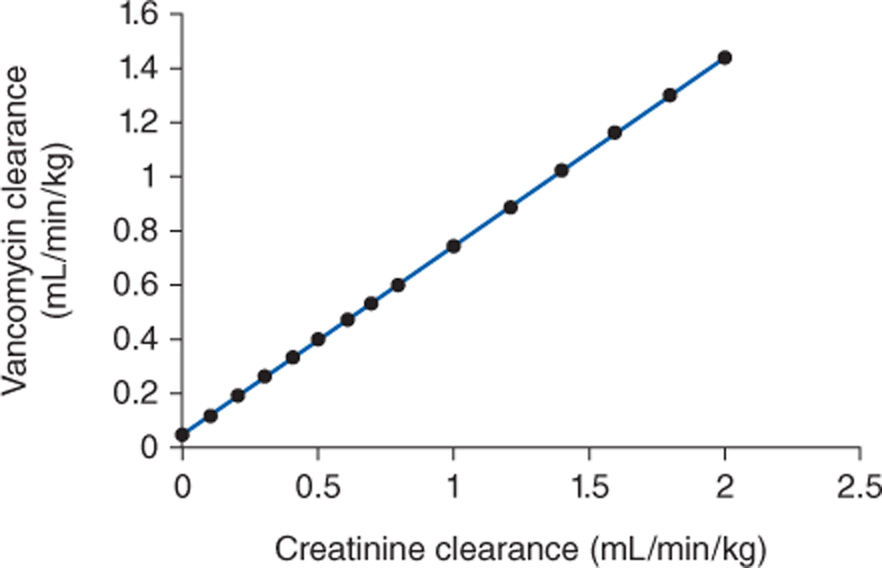
Because vancomycin is eliminated principally by glomerular filtration, renal dysfunction is the most important disease state that influences vancomycin pharmacokinetics.75–77 Vancomycin total clearance decreases proportionally to decreases in creatinine clearance (Figure 5-2).75 The relationship between renal function and vancomycin clearance forms the basis for initial dosage computation methods presented later in this chapter.
FIGURE 5-2 The clearance rate for vancomycin increases in proportion with creatinine clearance (CrCl). The equation for this relationship is Cl (in mL/min/kg) = 0.695(CrCl in mL/min/kg) + 0.05. This equation is used to estimate vancomycin clearance in patients for initial dosing purposes.
Major body burns (>30%-40% body surface area) can cause large changes in vancomycin pharmacokinetics.78,79 Forty-eight to 72 hours after a major burn, the basal metabolic rate of the patient increases to facilitate tissue repair. The increase in basal metabolic rate causes an increase in glomerular filtration rate that increases vancomycin clearance. Because of the increase in drug clearance, the average half-life for vancomycin in burn patients is 4 hours.
Patients with hematological malignancies have higher clearance rates and larger volumes of distribution for vancomycin.37,80–82 Because the changes increase both physiologic pharmacokinetic parameters simultaneously, the effect on half-life is variable, depending on whether the increase in volume of distribution or the increase in clearance predominates in an individual patient [t1/2 = (0.693 • V)/Cl].
Unlike with aminoglycosides, vancomycin pharmacokinetics in adult cystic fibrosis patients are not significantly different than typical values found in normal individuals.83
Obese individuals with normal serum creatinine concentrations have increased vancomycin clearance secondary to increased glomerular filtration rate and are best dosed with vancomycin using total body weight (TBW).73,74,84,85 The reason for the increased drug clearance is kidney hypertrophy that results in larger creatinine clearance rates. Volume of distribution does not significantly change with obesity and is best estimated using ideal body weight (IBW) in patients more than 30% overweight (>30% over IBW, V = 0.7 L/kg IBW).73,74,85 Because the primary pharmacokinetic change for vancomycin in obesity is increased drug clearance with a negligible change in volume of distribution, average half-life decreases to 3.3 hours [t1/2 = (0.693 • V)/Cl]. Some morbidly obese patients will require every 6- to 8-hour dosing to maintain vancomycin steady-state trough concentrations within the therapeutic range.73
Premature infants (gestational age 32 weeks) have a larger amount of body water compared to adults. However, vancomycin volume of distribution (V = 0.7 L/kg) is not greatly affected by these greater amounts of body water as is the case with aminoglycoside antibiotics. Kidneys are not completely developed at this early age so glomerular filtration and vancomycin clearance (15 mL/min) are decreased.86–89 A lower clearance rate with about the same volume of distribution as adults results in a longer average half-life for vancomycin in premature babies (10 hours). Full-term neonates (gestational age ∼40 weeks) have similar volumes of distribution for vancomycin compared to premature infants, but their vancomycin clearance rate is twice that found in infants born prematurely (30 mL/min). The increase in drug clearance is due to additional renal development that occurred in utero. The vancomycin half-life in full-term babies is about 7 hours. At about 3 months of age, vancomycin clearance has nearly doubled again (50 mL/min), resulting in a half-life of approximately 4 hours. The increase in vancomycin clearance continues through 4-8 years of age when clearance equals 130-160 mL/min while volume of distribution remains ∼0.7 L/kg so that half-life is 2-3 hours. At that time, vancomycin clearance and half-life gradually approach adult values as puberty approaches in children (∼12-14 years old).
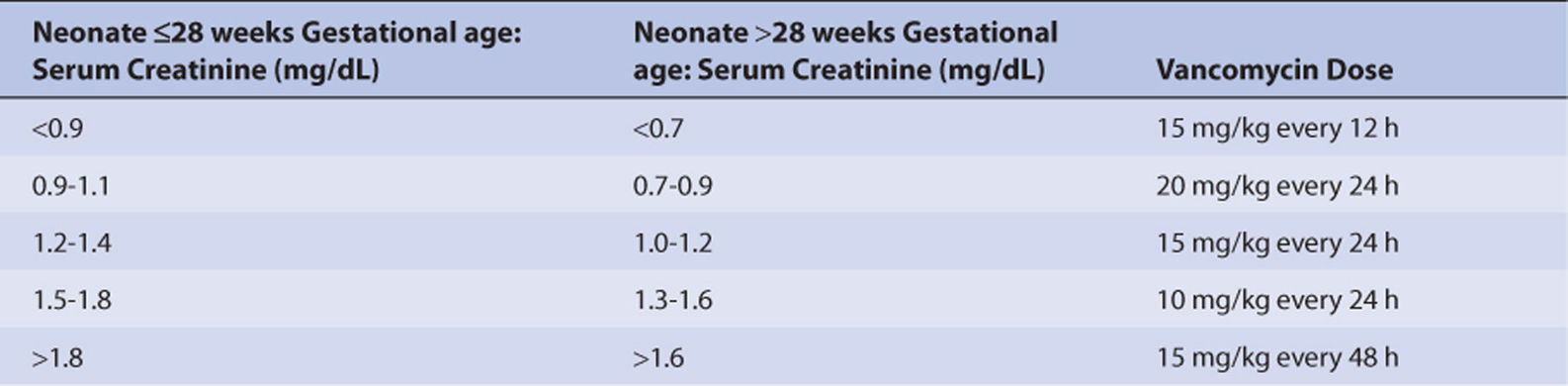
Intravenous vancomycin doses for neonates are based on renal function, weight, and age.90 Steady-state trough vancomycin serum concentrations are used to individualize doses:
Intravenous doses for older infants and children are 40-45 mg/kg/d given as three to four divided doses for mild to moderate infections (maximum dose, 1000-2000 mg/d) and 45-60 mg/kg/d given as three to four divided doses for severe infections (maximum dose, 2000-4000 mg/d).91 Steady-state trough vancomycin serum concentrations are used to individualize doses.
The effect that hemodialysis has on vancomycin pharmacokinetics depends on the type of artificial kidney used during the procedure. Vancomycin is a relatively large molecule with a moderate-sized volume of distribution and intermediate protein binding. These characteristics lead to poor hemodialysis removal from the body. The mean vancomycin half-life for patients with renal failure is 120-140 hours.28,77,92–98 Using traditional “low-flux” hemodialysis filters, an insignificant amount (<10%) of the total vancomycin body stores is removed during a 3- to 4-hour dialysis period.76,77 When hemodialysis is performed with a “high-flux” filter, vancomycin serum concentrations decrease with a half-life equal to 4-5 hours during the dialysis period, and 25%-45% of a vancomycin dose can be removed by the dialysis procedure. Compared to postdialysis values obtained immediately at the end of the procedure, serum concentrations of vancomycin can slowly increase or “rebound” over the next 2-6 hours by about 15%-35%. Postdialysis vancomycin serum concentrations to determine whether supplemental doses are needed should be measured after the rebound period in patients receiving hemodialysis with a high-flux filter.93–99
Because of the infusion rate–related side effects that occur with vancomycin, doses are sometimes given to hemodialysis patients directly into the dialysis tubing injection port 1-1.5 hours prior to the end of a session. In this case, it may be necessary to increase the vancomycin dose by 25%-35% if a high-flux hemofilter is used in order to compensate for the amount of drug removed during infusion.96,97 However, if a low-flux hemodialysis filter is being used during the infusion of vancomycin using this technique, such a low amount of the drug is lost that a dosage adjustment is not necessary.100
For patients with hemodynamic instability, a variant of hemodialysis known as sustained-low efficiency dialysis (SLED) or extended daily dialysis (EDD) can be performed where reduced blood flow and dialysate flow rates are used. To compensate for the reduced blood flow and dialysate flow rates (∼200 mL/min and ∼300 mL/min, respectively), the duration of dialysis is extended to 6-12 hours per day, and a high-flux filter is utilized most of the time. As one might expect, vancomycin is eliminated by this type of procedure, but the half-life during a dialysis period is 9-11 hours, and the amount of vancomycin removed is 20%-30%.94,101
Peritoneal dialysis removes only a negligible amount of vancomycin.102–104 Patients who develop peritonitis while receiving peritoneal dialysis can be treated by placing vancomycin into the dialysis fluid. Over a 6-hour dwell time, approximately 50% of a vancomycin dose (1000 mg in 2 L dialysis fluid) is absorbed from the peritoneal cavity in renal failure patients without peritonitis.102 Peritonitis causes inflammation of the peritoneal membrane, which facilitates absorption of vancomycin placed in the peritoneal dialysis fluid (up to 90% absorbed) and dialysis elimination of vancomycin from the body.104,105 Automated peritoneal dialysis is used at nighttime by some renal failure patients. This dialysis technique allows several exchanges of dialysis fluid during the night while the patient sleeps. In patients who use regular continuous ambulatory peritoneal dialysis (CAPD; dwell time 7-8 hours per exchange) augmented with automated peritoneal dialysis (dwell time 2-3 hours per exchange), vancomycin half-life is 12 hours during the automated procedure but 63 hours during CAPD.106
Continuous renal replacement therapy (CRRT) removes vancomycin from the body. The hemofiltration sieving coefficient for vancomycin is 0.80.107,108 In general, continuous venovenous hemofiltration (CVVH) and continuous arteriovenous hemofiltration (CAVH) provide a vancomycin clearance rate of about 20-30 mL/min, while continuous venovenous hemodialysis (CVVHD) and continuous venovenous hemodiafiltration (CVVHDF) can provide higher clearance rates.109–116 Recommended initial doses for critically ill patients with acute renal failure undergoing continuous venovenous hemofiltration (CVVH) are a loading dose of 15-20 mg/kg followed by 250-500 mg every 12 hours.117 For patients undergoing continuous arteriovenous hemofiltration (CAVH), the recommended initial dose is 500 mg every 24-48 hours.6 Because of pharmacokinetic variability, vancomycin concentrations should be measured in CRRT patients to ensure effective steady-state concentrations are maintained.
Extracorporeal membrane oxygenation (ECMO) is used in patients of all ages to aid in gas exchange for severe respiratory or cardiorespiratory failure. Vancomycin pharmacokinetics can be affected to a variable degree by ECMO, so steady-state vancomycin serum concentrations should be used to guide therapy in these patients.118–120 Cardiopulmonary bypass does not significantly influence the pharmacokinetics of vancomycin.121
DRUG INTERACTIONS
The most important drug interactions with vancomycin are pharmacodynamic, not pharmacokinetic, in nature. Coadministration of aminoglycoside antibiotics enhances the nephrotoxicity potential of vancomycin by twofold or more.50,122,123 Aminoglycosides can cause nephrotoxicity when administered alone. When an aminoglycoside and vancomycin are administered concurrently, serum creatinine concentrations should be monitored on a daily basis. Additionally, serum concentrations of the aminoglycoside, as well as vancomycin, should be measured. Patients receiving other nephrotoxic or ototoxic agents while taking vancomycin should be carefully monitored for adverse effects.
When vancomycin is administered to patients stabilized on warfarin therapy, the hypoprothrombinemic effect of the anticoagulant may be augmented.124 The mechanism of this interaction is unknown, but it resulted in a mean 45% increase in prothrombin time over baseline values when warfarin was given alone. Patients receiving warfarin therapy who require vancomycin treatment should have a baseline prothrombin time ratio (INR) measured before the antibiotic is administered and daily INR tests until it is certain that anticoagulation status is stable.
INITIAL DOSAGE DETERMINATION METHODS
Several methods to initiate vancomycin therapy are available. The Pharmacokinetic Dosing method is the most flexible of the techniques. It allows individualized target serum concentrations to be chosen for a patient, and each pharmacokinetic parameter can be customized to reflect specific disease states and conditions present in the patient. However, it is computationally intensive.58,125
Nomograms use the dosing concepts in the Pharmacokinetic Dosing method. However, in order to simplify calculations, they make simplifying assumptions. The Moellering nomogram is designed to achieve average steady-state concentrations equal to 15 μg/mL. Some clinicians find this approach confusing as target steady-state peak and trough concentrations are not stated by the nomogram. Because the computed dose provided by the nomogram is expressed in mg/kg/24 h, it can be difficult to determine the best dosage interval. However, once experience is gained with this approach, the Moellering nomogram computes doses similar, but not identical, to the Pharmacokinetic Dosing method. The Matzke nomogram is constructed to produce steady-state vancomycin peak and trough concentrations of 30 μg/mL and 7.5 μg/mL, respectively. When these target concentrations are acceptable, the Matzke nomogram computes doses that are very similar to those calculated by the Pharmacokinetic Dosing method. However, because the expected concentrations are below the contemporary therapeutic range for trough levels, the Matzke nomogram computes doses that may require some adjustment before administration to patients. To address the problem of a higher target steady-state target range, the Rybak nomogram was derived specifically to attain steady-state vancomycin trough concentrations of 15-20 μg/mL. However, its use is limited to CrClest between 40 and 109 mL/min and body weights of 50-110 kg.
Literature-based recommended dosing is a commonly used method to prescribe initial doses of vancomycin to pediatric patients. Doses are based on those that commonly produce steady-state concentrations within the therapeutic range, although there is a wide variation in the actual concentrations for a specific patient.
Pharmacokinetic Dosing Method
The goal of initial dosing of vancomycin is to compute the best dose possible for the patient given their set of disease states and conditions that influence vancomycin pharmacokinetics and the site and severity of the infection. In order to do this, pharmacokinetic parameters for the patient will be estimated using mean parameters measured in other individuals with similar disease state and condition profiles.
Clearance Estimate
Vancomycin is almost completely eliminated unchanged by the kidney, and there is a good relationship between creatinine clearance and vancomycin clearance (see Figure 5-2).75 This relationship permits the estimation of the vancomycin clearance for a patient, which can be used to calculate an initial dose of the drug. Mathematically, the equation for the straight line shown in Figure 5-2 is: Cl = 0.695(CrCl) + 0.05, where Cl is vancomycin clearance in mL/min/kg and CrCl is creatinine clearance in mL/min/kg. Because each clearance value is normalized for the patient’s weight, the estimated or measured creatinine clearance must be divided by the patient’s weight in kg before using it in the equation, and the resulting vancomycin clearance must be multiplied by the patient’s weight if the answer is needed in the units of mL/min. The weight factor that is used for all individuals, including obese patients, is total body weight (TBW).73,74,77,84,85 It is not possible to simply enter a patient’s creatinine clearance in mL/min and expect the resulting vancomycin clearance to have the units of mL/min with the idea that dividing the creatinine clearance by weight, then multiplying the vancomycin clearance by weight, mathematically cancels the weight factor out of the equation. The reason this does not work is that the y-intercept of the creatinine clearance/vancomycin clearance equation, which represents nonrenal vancomycin clearance, is in terms of mL/min/kg so mathematical cancellation of the weight factor is not possible. For example, the estimated vancomycin clearance for an individual with a creatinine clearance of 100 mL/min who weighs 70 kg is 1.04 mL/min/kg or 73 mL/min: Cl = 0.695[(100 mL/min)/70kg] + 0.05 = 1.04 mL/min/kg or 1.04 mL/min/kg • 70 kg = 73 mL/min. Taking the patient’s renal function into account when deriving an initial dose of vancomycin is the single most important characteristic to assess.
Volume of Distribution Estimate
The average volume of distribution of vancomycin is 0.7 L/kg for all patient groups except for those with hematologic malignancies, where the value is 1.05 L/kg.37,73,74,80–82 The weight factor that is used to calculate vancomycin volume of distribution for obese patients is ideal body weight (IBW).73,74,85 Thus, for an 80-kg patient, the estimated vancomycin volume of distribution would be 56 L: V = 0.7 L/kg • 80 kg = 56 L. For a 150-kg obese patient with an ideal body weight of 60 kg, the estimated vancomycin volume of distribution is 42 L: V = 0.7 L/kg • 60 kg = 42 L.
Elimination Rate Constant and Half-Life Estimates
The vancomycin elimination rate constant (ke) is computed using the estimated clearance and volume of distribution values for the drug in the following equation: ke = Cl/V. It is usually expressed using the unit of h−1. For example, for a patient with a vancomycin clearance equal to 1.04 mL/min/kg and a vancomycin volume of distribution equal to 0.7 L/kg, the elimination rate constant (in h−1) would be computed as follows: ke = (1.04 mL/min/kg • 60 min/h)/(0.7 L/kg • 1000 mL/L) = 0.089 h−1, where 60 min/h and 1000 mL/L are used as unit conversion factors for time and volume, respectively. Vancomycin half-life would be calculated using the equation that relates elimination rate constant and half-life: t1/2 = 0.693/ke = 0.693/0.089 h−1 = 7.8 h.
Selection of Appropriate Pharmacokinetic Model and Equations
When given by intravenous infusion over an hour, vancomycin serum concentrations follow a two- or three-compartment pharmacokinetic model (see Figure 5-1). After the end of infusion if a two-compartment model is followed, serum concentrations drop rapidly because of distribution of drug from blood to tissues (α or distribution phase). By about 30-60 minutes after the end of infusion, vancomycin serum concentrations decline more slowly, and the elimination rate constant for this portion of the concentration/time curve is one that varies with renal function (β or elimination phase). In patients whose vancomycin serum concentration/time curve follows a three-compartment model, an intermediate distribution phase is found between the α and β portions of the graph. While these models are important to understand conceptually, they cannot easily be used clinically because of their mathematical complexity. Because of this, the simpler one-compartment model is widely used and allows accurate dosage calculation when peak vancomycin serum concentrations are obtained after drug distribution is finished.73,77
Intravenously administered vancomycin is given over 1 hour as intermittent continuous infusions for doses between 1000-1500 mg. Because the drug has a long half-life relative to the infusion time (1 hour) and waiting time (0.5-1 hour) necessary to allow for distribution to complete before peak concentrations are obtained, little of the drug is eliminated during this 1.5- to 2-hour time period. Intravenous infusion pharmacokinetic equations that take into account the loss of drug during the infusion time are not generally needed because so little vancomycin is eliminated during the infusion and waiting time periods. Therefore, although the antibiotic is given as an intravenous infusion, intravenous bolus equations accurately predict peak vancomycin concentrations and are mathematically simpler.126 Because of these reasons, intravenous bolus equations are preferred by many clinicians to compute vancomycin doses (Table 5-2). Vancomycin steady-state peak (Cssmax) and trough (Cssmin) serum concentrations are chosen to treat the patient based on the type, site, and severity of infection as well as the infecting organism. Steady-state versions of one-compartment model intravenous bolus equations are as follows (see Table 5-2): Cssmax = (D/V)/(1 − e−keτ), Cssmin = Cssmaxe−keτ, where D is the antibiotic dose, V is the volume of distribution, ke is the elimination rate constant, t is time, and τ is the dosage interval.
TABLE 5-2A One-Compartment Model Equations Used With Vancomycin

C, drug serum concentration at time = t; D, dose; ke, elimination rate constant; n, number of administered doses; τ, dosage interval; V, volume of distribution.
TABLE 5-2B Pharmacokinetic Constant Computations Utilizing a One-Compartment Model Used With Vancomycin
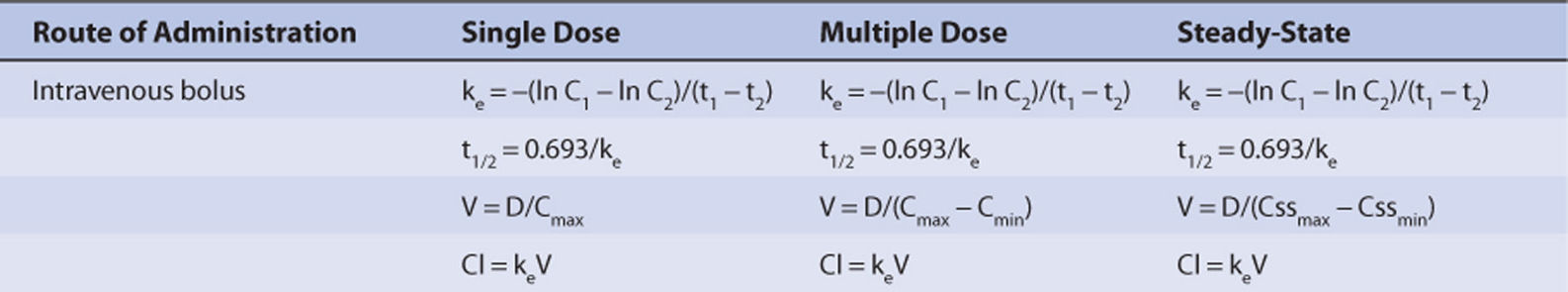
C0, concentration at time = 0; C1, drug serum concentration at time = t1; C2, drug serum concentration at time = t2; Cl, drug clearance; Cmin, predose trough concentration; Cmax, postdose peak concentration; D, dose; ke, elimination rate constant; t1/2, half-life; V, volume of distribution.
TABLE 5-2C Equations Used to Compute Individualized Dosage Regimens for Vancomycin

Cssmax, maximum steady-state concentration; Cssmin, minimum steady-state concentration; k0, continuous infusion rate; ke, elimination rate constant; V, volume of distribution.
Steady-State Concentration Selection
Vancomycin steady-state trough concentrations are selected based on site and severity of infection in addition to the infecting organism. Because of reports of therapeutic failures, current treatment guidelines recommend vancomycin steady-state trough concentrations equal to 10-15 μg/mL for lower-intensity dosing and 15-20 μg/mL for complicated infections due to MRSA, such as bacteremia, endocarditis, meningitis, osteomyelitis, severe skin infections, and hospital-acquired pneumonia.16–18 Steady-state vancomycin trough levels less than 10 μg/mL are discouraged due to the possibility of lower levels contributing to treatment failure or to the development of resistance. Whenever vancomycin doses are used that exceed steady-state trough concentrations of 20 μg/mL, serum creatinine concentrations and signs or symptoms of hearing or vestibular disturbance should be monitored daily to detect early signs of toxicity.
Steady-state peak vancomycin concentrations are chosen to provide adequate antibiotic penetration to the site of infection and to avoid adverse drug reactions. A commonly used range for this value is 30-50 μg/mL. In severe, life-threatening infections of the central nervous system, peak vancomycin serum concentrations as high as 60 μg/mL may be necessary to facilitate drug penetration. Whenever doses of vancomycin are used that exceed steady-state peak concentrations of 50 μg/mL, the patient should be monitored daily for early signs of ototoxicity (decreased hearing acuity in the conversational range, feeling of fullness or pressure in the ears, tinnitus, loss of equilibrium, headache, nausea, vomiting, vertigo, nystagmus, ataxia) or nephrotoxicity (serum creatinine concentrations).
Dosage Computation
The equations given in Table 5-2 are used to compute vancomycin doses.