Vaccines, Toxoids, and Other Immunobiologics
KEY CONCEPTS
![]() Live vaccines may confer life-long immunity but cannot be administered to the immunosuppressed.
Live vaccines may confer life-long immunity but cannot be administered to the immunosuppressed.
![]() Inactivated and subunit vaccines and toxoids often require multiple doses to protect from infection and generally booster doses are needed following the primary series.
Inactivated and subunit vaccines and toxoids often require multiple doses to protect from infection and generally booster doses are needed following the primary series.
![]() Children less than 2 years of age are unable to mount T-cell–independent immune responses that are elicited by polysaccharide vaccines.
Children less than 2 years of age are unable to mount T-cell–independent immune responses that are elicited by polysaccharide vaccines.
![]() Severely immunocompromised individuals should not receive live vaccines, and their responses to inactivated, polysaccharide, toxoid, and recombinant vaccines may be poor.
Severely immunocompromised individuals should not receive live vaccines, and their responses to inactivated, polysaccharide, toxoid, and recombinant vaccines may be poor.
![]() The childhood, adolescent, and adult immunization schedules are updated frequently and published annually. These documents can be used to develop an immunization plan for children.
The childhood, adolescent, and adult immunization schedules are updated frequently and published annually. These documents can be used to develop an immunization plan for children.
![]() Immunoglobulin (Ig) provides rapid postexposure protection from measles, hepatitis A, varicella, and other infections that wanes over time.
Immunoglobulin (Ig) provides rapid postexposure protection from measles, hepatitis A, varicella, and other infections that wanes over time.
![]() Ig adverse effects are often secondary to infusion rate. Slowing the IV infusion rate ameliorate chills, nausea, and fever that may develop during administration.
Ig adverse effects are often secondary to infusion rate. Slowing the IV infusion rate ameliorate chills, nausea, and fever that may develop during administration.
![]() Rho(D) Ig prevents Rh-negative mothers from mounting an immune response against hemolytic disease of the newborn. Hemolytic disease of the newborn results when Rh-negative mothers are sensitized to the Rh(D) antigen on the red blood cells of their fetuses.
Rho(D) Ig prevents Rh-negative mothers from mounting an immune response against hemolytic disease of the newborn. Hemolytic disease of the newborn results when Rh-negative mothers are sensitized to the Rh(D) antigen on the red blood cells of their fetuses.
Immunization is defined as rendering a person protected from an infectious agent. Immunity to an infectious agent can be acquired by exposure to the disease, by transfer of antibodies from mother to fetus, through administration of immunoglobulin (Ig), and from vaccination. Immunization is the process of introducing an antigen into the body to induce protection against the infectious agent without causing disease. An antigen is a substance that induces an immune response. An antibody produced by the humoral arm of the immune system usually is the response that is measured as evidence of successful vaccination. However, the cellular immune response, which is more difficult to measure, is also an important aspect of vaccine response.
This chapter introduces three groups of agents: vaccines, toxoids, and immune sera (together known as immunobiologics). Agents with a limited scope of use, such as agents for bioterrorism or travel, are beyond the scope of this chapter.
PRODUCTS USED TO IMMUNIZE
Vaccines and toxoids are separate and distinct products. However, both types of products induce active immunity—that is, immunity generated by a natural immunologic response to an antigen. Vaccines can be live attenuated or inactivated. Inactivated vaccines may consist of whole or split particles derived from the pathogen. Bacterial vaccines generally are killed whole bacteria or specific bacterial antigens or conjugates. Live-attenuated vaccines induce an immunologic response more consistent with that occurring with natural infection.![]() Because the organisms in live-attenuated vaccines undergo limited replication in the vaccinated individual after administration, they may confer lifelong immunity with one dose (as does a natural infection).
Because the organisms in live-attenuated vaccines undergo limited replication in the vaccinated individual after administration, they may confer lifelong immunity with one dose (as does a natural infection). ![]() Multiple doses of killed vaccines usually are needed to induce long-lasting, effective immunity. Additional doses at varying time intervals (booster doses) often are required to maintain immunity. Booster doses of such vaccines elicit memory responses from the B cells that produce immunoglobulin G (IgG). The immune system already has developed an array of antibodies to the antigen. Upon restimulation with a booster dose, the B cells, which produce the most specific antibodies against the antigen, are activated. Restimulation allows the most active antibodies against the antigen to be selected and maintained in the “immunologic memory.” Thus, the booster dose results in a rapid, intense antibody response that is long lasting. Inactivated vaccines also can differ in immunity potential, depending on their composition. For example, polysaccharide vaccines tend to be poorly immunogenic in infants, whereas protein–polysaccharide conjugated vaccines of the same antigen tend to be highly immunogenic (e.g., pneumococcal polysaccharide vaccine vs. pneumococcal conjugated vaccine).
Multiple doses of killed vaccines usually are needed to induce long-lasting, effective immunity. Additional doses at varying time intervals (booster doses) often are required to maintain immunity. Booster doses of such vaccines elicit memory responses from the B cells that produce immunoglobulin G (IgG). The immune system already has developed an array of antibodies to the antigen. Upon restimulation with a booster dose, the B cells, which produce the most specific antibodies against the antigen, are activated. Restimulation allows the most active antibodies against the antigen to be selected and maintained in the “immunologic memory.” Thus, the booster dose results in a rapid, intense antibody response that is long lasting. Inactivated vaccines also can differ in immunity potential, depending on their composition. For example, polysaccharide vaccines tend to be poorly immunogenic in infants, whereas protein–polysaccharide conjugated vaccines of the same antigen tend to be highly immunogenic (e.g., pneumococcal polysaccharide vaccine vs. pneumococcal conjugated vaccine). ![]() T-cell–independent immune response is made to polysaccharide antigens that stimulate B cells directly.1 There is no maturation or booster response with a T-cell–independent immune response, and children younger than 2 years cannot make this type of response. Protein–polysaccharide conjugate vaccines stimulate T cells and promote interactions between T cells and B cells when producing the protective immune responses consisting of immunologic memory and high-affinity IgG.
T-cell–independent immune response is made to polysaccharide antigens that stimulate B cells directly.1 There is no maturation or booster response with a T-cell–independent immune response, and children younger than 2 years cannot make this type of response. Protein–polysaccharide conjugate vaccines stimulate T cells and promote interactions between T cells and B cells when producing the protective immune responses consisting of immunologic memory and high-affinity IgG.
Toxoids are inactivated bacterial toxins that generally are combined with aluminum salts to enhance their antigenicity by prolonging antigen absorption and exposure. These adjuvants also increase local tissue irritation when injected. Toxoids stimulate the production of antibodies against the bacterial toxins rather than the infecting bacterial pathogens.
Immune sera are sterile solutions containing antibody derived from human (Ig) sources. Igs are derived from donor pools of blood plasma and are processed using cold ethanol fractionation in order to inactivate known potential pathogens. These sera are indicated for induction of passive immunity (temporary immunity to infection as a result of administration of antibodies not produced by the host; see Other Immunobiologics below).
In addition to the active component in an immunobiologic, other active and inert ingredients are often present. Suspending agents, such as water, saline, or complex fluids containing proteins (e.g., albumin), are used as the vehicle for the immunobiologic agent. Preservatives, stabilizers, and antibiotics may be added to help maintain the integrity of the product. Immunized individuals may respond with allergic reactions not to the immunobiologic agent itself but to the other components of the pharmaceutical preparation. Different manufacturers of the same immunobiologic may have different active and inert ingredients or different quantities of these ingredients in their products.
Certain vaccines manufactured by various companies are considered interchangeable. Hepatitis A, hepatitis B, and Haemophilus influenzae type b (Hib) conjugate vaccines from different manufacturers used for the primary series of three doses are considered interchangeable. It is preferable to use diphtheria, tetanus toxoids, and acellular pertussis (DTaP) vaccine from the same manufacturer to complete the entire primary series. However, immunization should not be delayed if the particular type of vaccine administered for the initial doses cannot be ascertained easily.1
In general, vaccines and toxoids must be kept refrigerated because breaking the “cold chain” may result in loss of potency. Varicella vaccine and zoster vaccines must be stored frozen. Immune sera generally should be kept refrigerated and not frozen except for lyophilized human IV immunoglobulin (IVIG), which can be stored at room temperature. Careful attention to appropriate storage of all vaccines and immunobiologics is absolutely imperative. Directions for appropriate storage can be found in the package inserts.
FACTORS AFFECTING RESPONSE TO IMMUNIZATION
Various factors are known to affect response to vaccines and toxoids. Viability of the antigen is an important factor (live attenuated vs. inactivated), as discussed previously. Total dose also is important because there seems to exist a threshold dose above which no further increase in antibody titer is seen. The interval between immunization doses, number of doses given, or both may change immune response to an agent. Among hepatitis B vaccine nonresponders, a significant proportion of individuals mount a vaccine response when given additional doses of vaccine.2 In contrast, additional doses of influenza vaccine are minimally effective in individuals with chronic illness.3 Generally, intervals longer than those recommended between vaccine doses do not reduce immune response.1
The route and site of administration of the immunobiologic are important. This is best illustrated by the hepatitis B vaccine, which elicits a satisfactory antibody response when given in the deltoid muscle but not a consistent response when administered in the gluteal area. Injections should be administered at a site with little likelihood of site damage. Immunobiologics containing adjuvants should be given into a muscle mass because they can cause irritation when given subcutaneously or intradermally.1
Host factors influence vaccine response. Immunocompromise, increasing age, underlying disease, and genetic background have been associated with poor response rates.1,4–6
VACCINE ADMINISTRATION
Subcutaneous injections should be administered into the thigh of infants and in the upper arm area over the triceps of older children and adults. A 5/8-inch, 25-gauge needle (0.508 mm × 1.6 cm) should be used, taking care not to administer the dose intradermally or intramuscularly (IM). For IM injection, the anterolateral aspect of the upper thigh (infants and toddlers) or the deltoid muscle of the upper arm (children and adults) should be used. When giving an IM injection to an adult weighing less than 60 kg, a 5/8-inch or 1-inch needle (1.6 cm or 2.5 cm) can be used. If a 5/8-inch needle (1.6 cm) is used, the skin over the injection site must be stretched tight and the needle must enter the skin at a 90° to assure that the needle reaches the muscle. A 1-inch needle (2.5 cm) should be used for adults who weigh 60 to 70 kg. Immunizers can choose either a 1-inch or 1½-inch needle (2.5 cm or 3.8 cm) for women who weigh 70 to 90 kg and for men who weigh 70 to 118 kg. For women weighing more than 90 kg and men who weigh more than 118 kg a 1½-inch needle (3.8 cm) must be used.1 The buttock should not be used because of the potential for inadequate immunologic response and the potential risk of injury to the sciatic nerve. When the buttock must be used (as for large doses of Ig), only the upper outer quadrant should be used with the needle inserted anteriorly. An influenza vaccine for intradermal administration is supplied in an injection device that reliably delivers the vaccine to the intradermal space. The intradermal injection is administered over the deltoid.7
The rotavirus vaccines are administered orally. The tube of vaccine should be squeezed inside the infant’s mouth toward the inner cheek until the dosing tube is empty. If the infant regurgitates or spits out the vaccine, readministration is not recommended.8
Live-attenuated influenza vaccine is administered intranasally.3 A specially designed sprayer is inserted just inside the nostril, and the dose is sprayed by depressing the plunger of the sprayer. The clip is removed from the plunger so that the second half of the dose can be administered into the other nostril. The vaccinated individual should breathe normally. The dose does not need to be repeated if the individual sneezes during or shortly after administration.
Questions often arise concerning the simultaneous administration of vaccines. In general, inactivated and live-attenuated vaccines can be administered simultaneously at separate sites. If two or more inactivated vaccines cannot be administered simultaneously, they can be administered without regard to spacing between doses. Inactivated and live vaccines can be administered simultaneously or, if they cannot be administered simultaneously, at any interval between doses, except for cholera (killed) and yellow fever (live) vaccines, which should be given at least 3 weeks apart. If live vaccines are not administered simultaneously, their administration should be separated by at least 4 weeks. Live viral vaccines may interfere with purified protein derivative response; thus, tuberculin testing should be postponed 4 to 6 weeks after administration of live-virus vaccine.1
Simultaneous administration of Ig and live-attenuated vaccines may inhibit host antibody response because of impairment of viral replication. A dose relationship exists between administration of Ig and inhibition of immune response to a vaccine (Table 102-1). Whole blood and other blood products containing antibodies may interfere with the response to the measles, mumps, and rubella (MMR) and varicella vaccines. In any patient, if vaccination with MMR or varicella is followed by emergency Ig administration, the vaccine can be repeated or seroconversion to viral antigens can be confirmed after sufficient time has elapsed (see Table 102-1). Ig does not interfere with the response to oral vaccines, zoster vaccine, or yellow fever vaccine.1
TABLE 102-1 Recommended Intervals Between Administration of Immunoglobulin and Measles-or Varicella-Containing Vaccine1
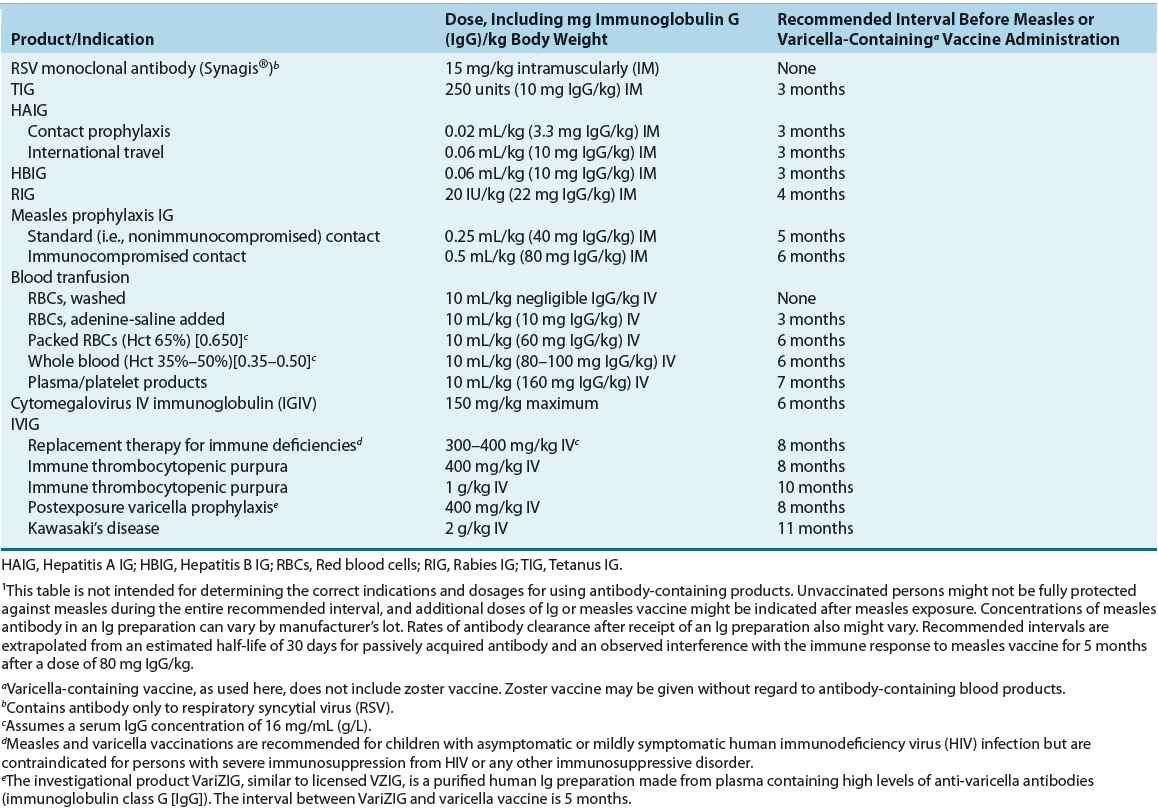
Inactivated vaccines and Igs may be administered simultaneously. However, different sites are recommended for killed vaccine and Ig administration.
VACCINE STORAGE
Appropriate storage is critical to maintaining the integrity of vaccines because improperly stored vaccines can fail to protect the individuals to whom they are administered. Refrigerator temperature is defined as between 2.2°C and 7.8°C (36°F to 46°F) and freezer temperature as –15°C (5°F) or colder. Inactivated vaccines are stored refrigerated. Varicella and zoster vaccines must be stored frozen. MMR vaccine can be stored in either the freezer or refrigerator. Live-attenuated influenza vaccine is stored in the refrigerator. Specific storage conditions for individual vaccines can be found in the package insert.
IMMUNIZATION OF SPECIAL POPULATIONS
Groups of individuals may have precautions to vaccines. Many precautions are temporary, and vaccines can be administered later.
Infants
The age of the recipient is an important determining factor in vaccine and toxoid response. In the first few months of life, maternal antibodies acquired via transplacental transfer during the third trimester of gestation protect an infant. However, the maternal antibodies also inhibit the immune response to live vaccines because the circulating antibodies neutralize the vaccine before the infant has the opportunity to mount an immune response. For this reason, live vaccines are not administered until maternal antibodies have waned, generally by infant age 12 months.1
Premature infants should be vaccinated at the same chronologic age using the same schedule and precautions for full-term infants. The full recommended doses of vaccines should be used, regardless of age or birth weight. Breastfed infants should be vaccinated according to standard pediatric schedules.
Pregnant Women and Postpartum Immunization
Most vaccines are pregnancy category C. As with most drugs, the vaccines are given this category assignment not because of a known risk to the fetus but because of lack of information. No birth defect has ever been attributed to vaccine exposure.1 For example, no cases of congenital rubella syndrome from inadvertent administration of rubella vaccine to a pregnant woman have ever been reported. Universal influenza immunization is recommended for women who will be or are pregnant during influenza season. Pregnant women should receive Tdap during the late second trimester or third trimester of pregnancy.9 Although live vaccines generally are avoided because of the theoretical risk of transmission of the vaccine organism to the fetus, inactivated vaccines may be administered to pregnant women when the benefits outweigh the risks.1 Hepatitis B, hepatitis A, meningococcal, inactivated polio, and pneumococcal polysaccharide vaccines should be administered to pregnant women who are at risk for contracting these infections.10
Administration of live vaccines, such as rubella or varicella, are deferred until postpartum and are routinely recommended for new mothers who do not have evidence of immunity prior to hospital discharge. These live vaccines can be administered without regard to administration of Rho(D) Ig in the postpartum period. Additionally, Tdap is recommended for all new mothers who have not received a Tdap before because household contacts are frequently implicated as the source of pertussis infection in a young infant.12
Immunocompromised Hosts
![]() Vaccination in compromised hosts (e.g., those with chronic disease, such as diabetes or connective tissue disease, alcoholics, or those with cancer or HIV disease) must be individualized based on the disease state and its treatment. In general, severely immunocompromised individuals should not receive live vaccines. Administration of other vaccines may be indicated, but responses may be lower than those mounted by healthy individuals, but may still confer protection.6
Vaccination in compromised hosts (e.g., those with chronic disease, such as diabetes or connective tissue disease, alcoholics, or those with cancer or HIV disease) must be individualized based on the disease state and its treatment. In general, severely immunocompromised individuals should not receive live vaccines. Administration of other vaccines may be indicated, but responses may be lower than those mounted by healthy individuals, but may still confer protection.6
Patients with chronic pulmonary, renal, hepatic, or metabolic disease who are not receiving immunosuppressants can receive both live-attenuated and killed vaccines and toxoids to induce active immunity. These patients often need higher doses of vaccines or more frequent dosing to induce immunity. Generally, immunization should be considered early in the course of the disease in an attempt to induce immunity at a point when the disease is less severe.
Patients with active malignant disease can receive killed vaccines or toxoids but should not be given live vaccines. The MMR vaccine is not contraindicated for close contacts, however. Live-virus vaccines can be administered to persons with leukemia who have not received chemotherapy for at least 3 months. Vaccines should be timed so that they do not coincide with the start of chemotherapy or radiation therapy.1 Zoster vaccine should be administered at least 2 weeks prior to the start of immunosuppressing therapy.11 Annual influenza vaccine should be administered 2 weeks prior to chemotherapy or between cycles.12 If vaccines cannot be given at least 2 weeks before the start of these therapies, immunization should be postponed until 3 months after the therapy has been completed. Passive immunization with Ig can be used in place of active immunization regardless of the history of immunization.
Glucocorticoids may cause suppressed responses to vaccines. For the purposes of immunization, the immunosuppressing dose of corticosteroids is prednisone 20 mg or more daily or 2 mg/kg daily, or an equivalent dose of another steroid, for at least 2 weeks. Patients receiving long-term, alternate-day steroid therapy with short-acting agents, administration of maintenance physiologic doses of steroids (e.g., 5 to 10 mg/day of prednisone) topical, aerosol, intraarticular, bursal, or tendon steroid injections require no special consideration for immunization. If patients have been receiving high-dose cortico-steroids or have had a course lasting longer than 2 weeks, then at least 1 month should pass before immunization with live-virus vaccines.1
Patients with HIV infection require special consideration. Responses to live and inactivated vaccines generally are suboptimal and decrease as the disease progresses because HIV produces defects in cell-mediated immunity and humoral immunity. The routinely recommended vaccines should be administered to children. Two doses of MMR vaccine should be administered at least 1 month apart as soon as possible after the first birthday. MMR and varicella virus should be administered only to children who have no or only moderate evidence of immunosuppression.1 Two doses of varicella vaccine separated by 3 months are recommended only for children with no evidence of immunosuppression. Adults should receive routinely recommended vaccines. Zoster vaccine may be administered to individuals with HIV infection who do not have clinical manifestations of AIDS and have CD4 counts >200/mm3 (>200 × 106/L).11
Solid Organ Transplant Patients
Organ transplantation has become routine treatment of end-stage organ disease of many causes. Although the number of organ transplants performed is severely limited by the availability of donor organs, survival of transplant recipients is increasing. Solid-organ transplant patients remain on immunosuppressive regimens for the rest of their lives. These immunosuppressive regimens result in a higher risk of infection and decrease the protection conferred by immunization.13
Whenever possible, transplant patients should be immunized prior to transplantation. Live vaccines generally are not given after transplantation. Posttransplantation diphtheria, tetanus, pneumococcal, and influenza vaccine responses are unpredictable. Decreased immune response has been documented following hepatitis B vaccine.
Hematopoietic Stem Cell Transplant Patients
Reimmunization of patients with hematopoietic stem cell transplantation is necessary because antibody concentrations wane rapidly. Annual influenza immunization may begin as soon as 6 months after successful engraftment. Reimmunization with inactivated vaccines should begin approximately 6 months after hematopoietic stem cell transplantation. Hematopoietic stem cell transplant recipients are at increased risk for fulminant infection with encapsulated bacteria, so 13-valent pneumococcal vaccine (PCV13), the 23-valent pneumococcal polysaccharide vaccine (PPSV23), meningococcal vaccine (MCV4), and Hib vaccines are recommended. MMR vaccine (MMR) can be administered at 24 months. Varicella vaccine is not routinely recommended but can be considered on a case-by-case basis. Immunization of household contacts and healthcare workers also is necessary.1
CONTRAINDICATIONS AND PRECAUTIONS
There are few contraindications to the use of vaccines except those outlined earlier. The contraindications include a history of anaphylactic reactions to the vaccine or a component of the vaccine. Unexplained encephalopathy occurring within 7 days of a dose of pertussis vaccine is a contraindication to future doses of pertussis vaccines. Immunosuppression and pregnancy are temporary contraindications to live vaccines. An interval of time must elapse based on the dose of Ig before a live vaccine can be administered (see Table 102-1). Precautions for DTaP administration include hypotonic hyporesponsive episode, fever of 40.5°C (104.9°F) or greater, crying lasting more than 3 hours within 48 hours of a previous dose, and seizures with or without fever within 3 days after a dose. A personal or family history of seizures is a precaution for receiving the combination MMR–varicella vaccine. Immunizers may choose to use MMR and varicella vaccines separately.1 Generally, mild-to-moderate local reactions, mild acute illnesses, concurrent antibiotic use, prematurity, family history of adverse events, diarrhea, and lactation or breastfeeding are not contraindications to immunization.
OBTAINING AN IMMUNIZATION HISTORY
An immunization history should be obtained from every patient, regardless of the reason for the healthcare visit. Ideally, any history provided by the patient from memory should be verified by reviewing the patient’s personal written immunization record or a database that contains the complete immunization history. State-based or other public health jurisdiction-based immunization information systems (IIS), also called immunization registries, have been developed to improve immunization coverage by allowing healthcare providers access to records at any contact with the healthcare system. Registries are aimed primarily at facilitation of childhood immunization records.14 If an official written record is not available, patient characteristics (e.g., military service, travel history, and occupation) may provide clues to the immunization history. Serologic testing for immunity against certain diseases can provide specific information but is used routinely for only a few selected diseases (e.g., measles, rubella, hepatitis A and B, and varicella) and selected circumstances (e.g., employment in a healthcare facility). If a written record does not exist, one should be generated at the time of initiation of immunization. Patients without a written record should be considered susceptible, and an immunization program started and completed unless a serious adverse reaction occurs. As a general rule, the risks associated with overimmunization are minimal relative to the risks associated with contracting vaccine-preventable diseases.1
Every healthcare visit, regardless of its purpose, should be viewed as an opportunity to review a patient’s immunization status and to administer needed vaccines. Immunization is perhaps the most cost-effective health intervention available. Each visit should include assessment of individuals’ vaccine needs, administration of indicated vaccines, and documentation of immunization histories. The outcome measurement of what percentage of patients in a particular practice site is completely immunized is extremely important because the benefits of optimal vaccine use extend beyond the individual patient to the public as a whole.
NATIONAL VACCINE INJURY COMPENSATION PROGRAM
The National Childhood Vaccine Injury Act of 1986 was passed by the U.S. Congress in response to reports of vaccine side effects and liability concerns of vaccine manufacturers and healthcare providers. With vaccine safety being questioned and manufacturers ceasing the development and marketing of vaccines, the National Vaccine Injury Compensation Program was implemented to offer a no-fault alternative means to compensate individuals for injury following vaccination. The program offers liability protection to manufacturers and an efficient means of recovering damages for individuals potentially injured by vaccines. Compensation for vaccine-related injuries is outlined in the Health Resources and Services Administration’s Vaccine Injury Table (http://www.hrsa.gov/vaccinecompensation/vaccinetable.html). Healthcare providers must report all events requiring medical attention within 30 days of vaccination to the Vaccine Adverse Event Reporting System (VAERS), which serves as a central depot for vaccine-related adverse effects. Only a temporal association between the adverse event and vaccine administration needs to be made. No adverse event rates can be determined because only the number of adverse events reported is known; the number of vaccines administered is not known. This database can be used to survey for changes in the frequencies of adverse events, to evaluate risk factors for adverse events, and to find rare adverse events.15 VAERS report forms can be obtained by calling 1-800-822-7967, or reports can be made online at https://vaers.hhs.gov/esub/index.
USE OF VACCINES AND TOXOIDS
The Advisory Committee on Immunization Practices (ACIP) makes recommendations for use of vaccines for the United States. Other professional organizations, for example, the American Academy of Pediatrics, the American Academy of Family Physicians, or the American College of Obstetrics and Gynecology, publish guidelines. Usually, these guidelines are the same as those issued by the ACIP or the groups try to reconcile their recommendations.
![]() The appendices show the recommended schedules for routine immunization of children and adults. The latest vaccine schedules can be found at http://www.cdc.gov/vaccines/schedules/hcp/index.html. All states require children to be fully immunized prior to entering elementary school; however, optimal protection is achieved by immunizing at the recommended ages, which requires special attention to children younger than 2 years. Adults and adolescents also require vaccination and often are unaware of this need. An early adolescent preventive health visit is recommended. This visit is an opportunity to catch up on missed immunizations and to administer meningococcal conjugate, Tdap, and human papillomavirus (HPV) vaccines. All individuals older than 6 months of age should receive an annual seasonal influenza vaccine. Adults should receive routine tetanus–diphtheria (Td) or Tdap boosters and be immune to measles, mumps, rubella, and varicella by either immunization or history of infection. Older adults need zoster vaccine after age 60 years, and pneumococcal polysaccharide vaccine after age 65 years. Certain individuals with conditions or lifestyles that put them at high risk for vaccine-preventable diseases also should be immunized as described in the following text and outlined in the immunization schedules in the appendices.
The appendices show the recommended schedules for routine immunization of children and adults. The latest vaccine schedules can be found at http://www.cdc.gov/vaccines/schedules/hcp/index.html. All states require children to be fully immunized prior to entering elementary school; however, optimal protection is achieved by immunizing at the recommended ages, which requires special attention to children younger than 2 years. Adults and adolescents also require vaccination and often are unaware of this need. An early adolescent preventive health visit is recommended. This visit is an opportunity to catch up on missed immunizations and to administer meningococcal conjugate, Tdap, and human papillomavirus (HPV) vaccines. All individuals older than 6 months of age should receive an annual seasonal influenza vaccine. Adults should receive routine tetanus–diphtheria (Td) or Tdap boosters and be immune to measles, mumps, rubella, and varicella by either immunization or history of infection. Older adults need zoster vaccine after age 60 years, and pneumococcal polysaccharide vaccine after age 65 years. Certain individuals with conditions or lifestyles that put them at high risk for vaccine-preventable diseases also should be immunized as described in the following text and outlined in the immunization schedules in the appendices.
TOXOIDS
Diphtheria Toxoid Adsorbed
Diphtheria is an acute illness caused by the toxin released by a Corynebacterium diphtheriae infection. The toxin inhibits cellular protein synthesis, and membranes form on mucosal surfaces. Systemic toxemia can result in myocarditis, neuritis, and thrombocytopenia. Membrane formation can cause respiratory obstruction, and significant toxin absorption can lead to severe illness and death.
Diphtheria toxoid adsorbed is a sterile suspension of modified toxins of C. diphtheriae that induces immunity against the exotoxin of this organism. Two strengths of diphtheria toxoid are available in the United States: pediatric strength (D) and adult strength (d), which contains less antigen. The widespread use of diphtheria toxoid essentially has eliminated diphtheria from the United States.
Primary immunization with diphtheria toxoid (D) is indicated for children older than 6 weeks. The toxoid is given in combination with tetanus toxoid and acellular pertussis vaccine (as DTaP or in combination with additional childhood vaccines that have been licensed to decrease the number of injections required to complete the childhood immunization recommendations) at age 2, 4, and 6 months. Additional doses are given at age 15 to 18 months and again at age 4 to 6 years.16 Completing the primary diphtheria toxoid immunization series usually induces immunity of at least 10 years’ duration in 90% of persons. Booster doses should be given every 10 years.
For unimmunized adults, a complete three-dose series of diphtheria toxoid should be administered, with the first two doses given at least 4 weeks apart and the third dose given 6 to 12 months after the second. The combined Td preparation is recommended for adults because it contains less diphtheria toxoid than the pediatric dose and is associated with fewer reactions to the diphtheria component. One of the vaccine doses in this series should be Tdap. All adults should receive booster doses of Td every 10 years.17 Adverse effects of diphtheria toxoid include mild-to-moderate tenderness, erythema, and induration at the injection site. Systemic reactions occur very rarely.
Tetanus Toxoid Adsorbed and Tetanus Immunoglobulin
Tetanus is a severe acute illness caused by the exotoxin of Clostridium tetani. Tetanus is the only vaccine-preventable disease that is not contagious. It is acquired from the environment. Sustained muscle contractions are characteristic of tetanus. Tetanus toxin interferes with neurotransmitters that promote muscle relaxation, leading to continuous muscle spasms. Death can be due to the tetanus toxin itself or secondary to a complication such as aspiration pneumonia, dysregulation of the autonomic nervous system, or pulmonary embolism.
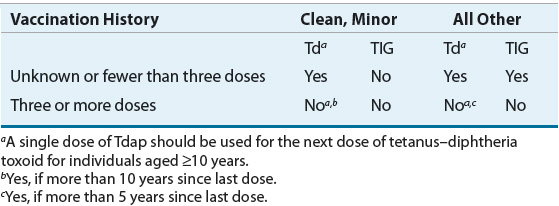
Tetanus toxoid adsorbed (adsorbed onto aluminum hydroxide, phosphate, or potassium sulfate to increase antigenicity) is a sterile suspension of the toxoid derived from C. tetani. A series of three 0.5-mL doses of tetanus toxoid elicits protection in virtually all individuals. Primary vaccination provides protection for at least 10 years.17,18 Additional doses of tetanus toxoid (combined with diphtheria toxoid, i.e., Td) are recommended as part of wound management if a patient has not received a dose of tetanus toxoid within the preceding 5 years.19 For minor or clean wounds, no dose is given. Table 102-2 summarizes these recommendations. Tetanus Ig should be given to individuals who have received fewer than three doses of tetanus toxoid and have more serious wounds. It can be administered with tetanus toxoid, provided that separate syringes and separate injection sites are used.
In children, primary immunization against tetanus usually is offered in conjunction with diphtheria and pertussis vaccination (using DTaP or a combination vaccine that includes other antigens used to decrease the number of injections to complete the childhood immunization schedule). A 0.5-mL dose is recommended at age 2, 4, 6, and 15 to 18 months, but the first dose can be administered as early as age 6 weeks.16 In children 7 years and older and in adults who have not been immunized previously, a series of three 0.5-mL doses of a tetanus toxoid-containing vaccine is administered IM initially. The first two doses are given 1 to 2 months apart, and the third dose is recommended at 6 to 12 months after the second dose. Boosters are recommended every 10 years, and unless there is contraindication to diphtheria toxoid, Td should be used.16,20 Tetanus toxoid can be given simultaneously with other killed and live vaccines, and, if indicated, it can be given to immunosuppressed patients.
Adverse reactions to tetanus toxoid include mild-to-moderate local reactions at the injection site, such as warmth, erythema, and induration. Occasionally, a nodule at the injection site develops and remains for a few weeks. Very rare major local reactions occur within 2 to 8 hours of administration to patients with high serum tetanus antitoxin levels. This type of reaction is indicative of high preexisting antibody concentrations, and additional doses of toxoid should not be given any sooner than 10 years. Local reactions do not limit the use of the toxoid for further dosing.21
Tetanus Ig is a sterile, concentrated, nonpyrogenic solution of Igs prepared from hyperimmunized humans. It is used to provide passive immunity to tetanus after the occurrence of traumatic wounds in nonimmunized or suboptimally immunized persons (see Table 102-2).22 A dose of 250 to 500 units IM should be administered. When administered with tetanus toxoid, separate sites for administration should be used. Tetanus Ig also is used for treatment of tetanus. In this setting, a single dose of 3,000 to 6,000 units IM is administered.
Adverse effects of tetanus Ig include pain, tenderness, erythema, and muscle stiffness at the injection site, which may persist for several hours. Systemic reactions occur rarely. IV administration has been associated with severe adverse reactions and is not recommended.
VACCINES
Haemophilus Influenzae Type B Vaccines
Before 1995, Hib was responsible for thousands of cases of serious illnesses (e.g., meningitis, epiglottitis, pneumonia, sepsis, and septic arthritis). The incidence of Hib disease has declined more than 99% since introduction of the conjugate vaccines based on the organism’s capsular substance, polyribosylribitol phosphate (PRP).23
The Hib vaccines used are conjugate products consisting of either a polysaccharide or an oligosaccharide of PRP covalently linked to a protein carrier. The protein carrier is important because it provides for T-lymphocyte–dependent immunologic response, whereas earlier Hib vaccines that consisted of only unconjugated PRP elicited a response that was T-cell independent. T-cell involvement in the response provides for (a) a greater antibody response regardless of the age of the patient receiving the vaccine, (b) immunologic response at an earlier age (including infants), and (c) a booster effect on subsequent exposure to the Hib capsule, whether through revaccination or natural exposure. The protein carrier is not considered a vaccine and should not be substituted for immunization against tetanus, diphtheria, or Neisseria meningitidis.
Hib conjugate vaccines are indicated for routine use in all infants and children younger than 5 years. Multiple products in various combinations are available for use in infants and children of different ages. The primary series of Hib vaccination consists of a 0.5-mL IM dose at ages 2, 4, and 6 months. If Hib PRP-OMP (outer membrane protein of Neisseria meningitides as the protein conjugate) is being used, the primary series consists of doses given at ages 2 and 4 months. The series should not be initiated in an infant younger than 6 weeks. Although use of one product for the entire primary series is desirable, adequate protection is achieved even when different products are used during the initial doses. Following the primary series, a booster dose is recommended at age 12 to 15 months. Any of the Hib conjugate vaccines are suitable for the booster dose regardless of which conjugate was used for the primary series of doses.23
Schedules are more complex for infants who do not begin Hib immunization at the recommended age or who have fallen behind in the immunization schedule. For infants 7 to 11 months of age who have not been vaccinated, three doses of Hib vaccine should be given: two doses spaced 4 weeks apart and then a booster dose at age 12 to 15 months (but at least 8 weeks since the second dose). For unvaccinated children ages 12 to 14 months, two doses should be given, with an interval of 2 months between doses. In a child older than 15 months, a single dose of any of the vaccine preparations is indicated.16
Vaccines for Hib are recommended for routine use only for patients up to age 59 months; beyond this age, most individuals will have natural immunity to Hib infection. Patients with certain underlying conditions (e.g., HIV infection, IgG2 subclass deficiency, sickle cell disease, splenectomy, and hematopoietic stem cell transplants and those receiving chemotherapy for malignancies) are at higher than normal risk for Hib infection, and use of at least one dose of vaccine in these patients should be considered, although efficacy data in most of these situations are lacking.1,16,20
Adverse reactions to the Hib vaccine are uncommon. Erythema and induration at the injection site occur in approximately 5% to 30% of children and resolve within 12 to 24 hours. Fever, diarrhea, and vomiting are reported occasionally.23
Hepatitis Vaccines
Information on vaccination for viral hepatitis is given in Chapter 26.
Human Papillomavirus Vaccine
HPV infections are the most common sexually transmitted infections, with the highest prevalence of infection in sexually active young adults.24 Although more than 120 different HPV types have been identified, at least 40 different types of HPV infect the anogenital tract. These 40 different viruses are grouped into low-risk and high-risk types. Low-risk types can cause genital warts and mild abnormalities on Papanicolaou (Pap) tests. Ninety percent of all cases of genital warts are caused by types 6 and 11. As many as 18 types are considered high risk as they have the ability to penetrate the nucleus of an epithelial cell to transform it to a precancerous cell. They cause abnormal Pap test results and may lead to cancer of the cervix, vulva, vagina, anus, or penis. Types 16 and 18 cause about 70% of all cervical cancers. High-risk HPV infections are necessary but not sufficient for the development of cervical cancer and for the majority of other anogenital and oral squamous cell cancers.
A bivalent HPV (Cervarix, GSK) containing virus-like particles for types 16 and 18 was licensed in late 2009. The quadrivalent vaccine (Gardasil, Merck Vaccines) is directed against cervical cancer-causing types 16 and 18 and types 6 and 11. ACIP recommends either of these HPV vaccine preparations for the prevention of cervical cancer and precancerous lesions. No head-to-head comparison of these vaccines is available, but both vaccines are very efficacious for the prevention of precancerous lesions caused by types 16 and 18.25 Both vaccines offer some protection against oncogenic nonvaccine strains too.26–28 Both vaccines are administered as a three-dose series using a harmonized schedule of 0, 1 to 2, and 6 months. The vaccines are recommended for females aged 11 to 12 years and for all females aged 13 to 26 years. Although administration of these vaccines before sexual debut is preferable, the vaccines can be administered without regard to history of sexual activity.25
The quadrivalent HPV vaccine is licensed for the prevention of genital warts, anal cancer, and precancerous lesions in males aged 9 to 26 years. Types 6 and 11 cause approximately 90% of genital warts—about 250,000 cases in males each year. Approximately, 7,000 cancers in males are associated with HPV types 16 or 18 infection annually. Additional clinical information showing HPV4 to be effective in the prevention of anal intraepithelial neoplasia (a precursor to anal cancer) among men who have sex with men (MSM) was published.29 MSM are at a higher risk for infection with HPV, genital warts, and anal cancer.30 The incidence of cancers associated with HPV is higher among MSM, and the rate of anal cancer among MSM continues to rise.29,30 The ACIP recommends routine HPV4 vaccine series for males at age 11 to 12 years. Vaccination of males aged 13 to 21 years who have not been vaccinated or who have not completed all three doses of the HPV4 series is also recommended. In addition, males aged 21 to 26 years may receive HPV4. Routine vaccination with HPV4 for MSM through age 26 years is also recommended.30
The vaccines are well tolerated, with injection-site reactions and systemic reactions (e.g., headache and fatigue) occurring as commonly in immunized individuals as in the groups receiving placebo. Although syncope is possible with any immunization, the target population of adolescents and young adults has a higher incidence of syncope, including with administration of the HPV vaccine.25
These effective vaccines is an important advance, but the need for a Pap test for cervical cancer screening remains. Surveillance for duration of protection conferred by the vaccine series is ongoing; the need for future booster doses is not yet known.
Influenza Virus Vaccine
Information on vaccination for influenza is given in Chapter 87.
Measles Vaccine
Measles (rubeola) is a highly contagious viral illness characterized by rash and high fever. Complications of measles infections include severe diarrhea, otitis media, pneumonia, and encephalitis. Measles results in one to two deaths per 1,000 cases, with a much higher death rate in developing countries. With widespread vaccination, measles is on the verge of elimination from the Western Hemisphere.
The measles vaccine is a live-attenuated viral vaccine that produces a subclinical, noncommunicable infection. Approximately 95% of vaccine recipients mount a protective immune response after a single dose, and most individuals are protected for life.25 Most persons who do not respond to the first dose of measles vaccine will respond after receiving a second dose, and this forms the basis for the two-dose vaccine strategy that was implemented in the United States in 1989.
The measles vaccine is administered subcutaneously as a 0.5-mL dose in the arm (or in the thigh if the patient is younger than 15 months). The vaccine is administered routinely for primary immunization to persons 12 to 15 months of age. Two combinations of measles containing vaccines are available—measles–mumps–rubella (MMR) or measles–mumps–rubella–varicella (MMRV). The measles vaccine is not administered earlier than 12 months (except in certain outbreak circumstances) because persisting maternal antibody that was acquired transplacentally late in gestation can neutralize the vaccine virus before the vaccinated person can mount an immune response. A second dose of measles-containing vaccine is recommended when children are 4 to 6 years old.16 The second dose of vaccine results in seroconversion in 95% of individuals who were first-dose nonresponders.
Measles-containing vaccine should not be given to pregnant women or immunosuppressed patients. The one exception is HIV-infected patients, who are at very high risk for severe complications if they develop measles. Persons with HIV infection who have never had measles or have never been vaccinated against it should be given measles-containing vaccine unless there is evidence of severe immunosuppression. The second dose should be given 1 month later rather than waiting for entry to school.31,32
Recent administration of Ig interferes with measles vaccine response, so the recommended interval between the Ig and vaccine is determined by the dose of Ig (see Table 102-1).1 Live vaccines not administered during the same visit must be delayed for at least 30 days following measles or MMR vaccine. Live measles vaccine may suppress a positive tuberculin skin test for up to 6 weeks postadministration.1 Mild febrile illness and upper respiratory tract infections are not contraindications to vaccination.
Measles vaccination is indicated in all persons born after 1956 or in those who lack documentation of wild virus infection by either history or antibody titers. Two doses of a measles-containing vaccine are required for college students and healthcare workers who were born in 1957 or later. If two doses are needed (the person has never been vaccinated), the doses should be given at least 1 month apart.16,20
The measles vaccine has an excellent safety record. The most common side effect following vaccination is fever, which occurs in 5% to 15% of vaccinees. Transient generalized rash may occur in approximately 5% of vaccine recipients. These reactions generally appear 5 to 12 days postvaccination and last 2 to 5 days. Other adverse effects, such as headache, cough, sore throat, eye pain, malaise, and transient thrombocytopenia, occur less frequently.
Meningococcal Polysaccharide and Conjugate Vaccines
N. meningitidis is a leading cause of meningitis and sepsis in children and young adults in the United States. The infection is transmitted by respiratory droplets from infected individuals and asymptomatic carriers. Symptoms include severe headache, sensitivity to light, stiff neck, nausea and vomiting, and high fever. Mortality occurs in 24 to 48 hours following onset of symptoms in 10% to 13% of infected individuals.33
Two meningococcal conjugate vaccines combining the same serotypes are licensed for use in individuals aged 9 months to 55 years old (Menactra®, Sanofi-Pasteur) or 2 to 55 years old (Menveo®, Novartis). A quadrivalent vaccine containing capsular polysaccharides for serotypes A, C, Y, and W-135 (Menimmune®, Sanofi-Pasteur) has been available since the early 1970s. Although infections with serogroup B occur at rates that vary with age, it has not been incorporated into the vaccine because group B polysaccharide is not immunogenic. The meningococcal conjugate vaccine is indicated in adolescents at ages 11 to 12 years with a second dose at age 16 years. The vaccine is also recommended for high-risk populations, such as those exposed to the disease, those in the midst of uncontrolled outbreaks, travelers to areas with epidemic or hyperendemic meningococcal disease, and individuals who have terminal complement component deficiencies or asplenia. Reimmunization at 5-year intervals is recommended for individuals who are at high risk.34 The polysaccharide vaccine should be reserved for those older than 55 years of age who require immunization.
Injection-site reactions are the most common adverse effects following administration of either the meningococcal conjugate or polysaccharide vaccine.
Mumps Vaccine
Mumps is a viral illness that classically causes bilateral parotitis 16 to 18 days after exposure. Fever, headache, malaise, myalgia, and anorexia may precede the parotitis. Serious complications are rare but more common in adults.
The mumps vaccine is a lyophilized live-attenuated vaccine prepared from chick embryo cultures. Each 0.5-mL dose of the vaccine also contains neomycin 25 mcg. The vaccine is available in combinations with measles, rubella (as MMR), and varicella (MMRV) vaccines.
The vaccine is administered as a 0.5-mL subcutaneous injection in the upper arm. Dosing recommendations coincide with those for measles vaccine, with the first dose administered at age 12 to 15 months and the second dose prior to the child’s entry into elementary school. Two doses of mumps-containing vaccine are recommended for school-aged children, international travelers, students in post-high school educational institutions, and healthcare workers born after 1956.31 A single dose of vaccine is acceptable documentation of immunity to mumps for other adults considered at lower risk of mumps infection, including adults born after 1956 and those with an uncertain history of wild virus infection.
Mumps vaccine should not be given to pregnant women or immunosuppressed patients.1 The effect of Ig preparations on mumps vaccine response is unknown, but the response to measles, rubella, and varicella is compromised if the vaccine is administered after Igs. The recommended interval between the Ig and vaccine is determined by the dose of Ig (see Table 102-1).1 The vaccine should not be given to individuals with anaphylactic reactions to neomycin.
Serious adverse reactions to the vaccine are reported rarely. Parotitis, rash, pruritus, and purpura occur rarely. Local reactions, including soreness, burning, and stinging, may occur at the injection site.
Pertussis Vaccine
Pertussis is caused by a bacterial infection with Bordetella pertussis. The infection starts with signs and symptoms of an acute respiratory infection, called the catarrhal stage. The coughing spells manifest about a week later. Typically, young children will have the characteristic whoop as they struggle to inhale while coughing. Adolescents and adults are more likely to have prolonged periods of coughing. Pertussis can affect any age group, but young infants are at much higher risk for pneumonia, seizures, brain damage, and death. Their rate of hospitalization is much higher than for other age groups. The individual is contagious during the catarrhal stage and the first two weeks of the cough.17,18
Acellular pertussis vaccines contain components of the B. pertussis organism. All acellular vaccines contain pertussis toxin, and some contain one or more additional bacterial components (e.g., filamentous hemagglutinin, pertactin [a 69-kDa outer membrane protein], and fimbriae types 2 and 3). Acellular pertussis vaccine is recommended for all doses of the pertussis schedule at 2, 4, 6, and 15 to 18 months of age. A fifth dose of pertussis vaccine is given to children 4 to 6 years of age.16 Pertussis vaccine is administered in combination with diphtheria and tetanus (DTaP). Administration of an acellular pertussis-containing vaccine is also recommended for adolescents once between ages 11 and 18 years. In addition, they should receive a pertussis-containing vaccine with their next dose of Td toxoids.17,18 Special attention is warranted for the immunization of individuals who have close contact with young infants. Tdap should be administered to women in their late second or third trimester of pregnancy. Tdap should also be administered to all close contacts, including household contacts and out of home care providers.9
Local administration site reactions are relatively common. Systemic reactions, such as moderate fever, occur in 3% to 5% of vaccinees. Very rarely, high fever, febrile seizures, persistent crying spells, and hypotonic hyporesponsive episodes occur following vaccination. Allergy to a vaccine component and encephalopathy without known cause within 7 days of a pertussis vaccine are contraindications to future doses of vaccine.
Pneumococcal Vaccines
Streptococcus pneumoniae is a common pathogen with a range of manifestations, including asymptomatic upper respiratory tract colonization, sinusitis, acute otitis media, pharyngitis, pneumonia, meningitis, and bacteremia. Rates of invasive infections are highest in children younger than 2 years and in the elderly.35,36 Invasive pneumococcal infections cause approximately 40,000 deaths annually. Most of the deaths occur in the elderly or in those with underlying medical conditions. Approximately half the deaths could be preventable by vaccine. Two pneumococcal vaccine preparations, PCV13 and 23-valent pneumococcal polysaccharide vaccine (PPV23) are available. The vaccines have different indications and are not interchangeable.
Pneumococcal Polysaccharide Vaccine
Pneumococcal polysaccharide vaccine (Pneumovax 23) is a mixture of highly purified capsular polysaccharides from 23 of the most prevalent or invasive types of S. pneumoniae seen in the United States. Serotypes included are 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F. These 23 types represent 85% to 90% of all blood isolates and 85% of pneumococcal isolates from other generally sterile sites seen in the United States. The vaccine is administered IM or sub-cutaneously as a single 0.5-mL dose. Each 0.5-mL dose of vaccine contains 25 mcg of each polysaccharide type dissolved in isotonic saline solution (for a total of 575 mcg polysaccharide) and 0.25% phenol as preservative.
PPSV23 is recommended for the following individuals:37
1. Persons 65 years and older (if an individual received vaccine more than 5 years earlier and was younger than 65 years at the time of administration, revaccination should be given).
2. Persons aged 2 to 64 years with a chronic illness (congestive heart failure, cardiomyopathy, chronic pulmonary disease, diabetes, alcoholism, and liver disease).
3. Persons aged 2 to 64 years with functional or anatomic asplenia (when splenectomy is planned, PPSV23 should be given at least 2 weeks before surgery; a single revaccination is recommended at 5 years in subjects older than 10 years and at 3 years in subjects younger than 10 years).
4. Persons aged 19 to 64 years who smoke cigarettes or have asthma.
5. Persons with cochlear implants.
PPSV23 is recommended for immunocompromised persons 2 years and older with (a) HIV infection, (b) leukemia, (c) lymphoma, (d) Hodgkin’s disease, (e) multiple myeloma, (f) generalized malignancy, (g) chronic renal failure or nephrotic syndrome, (h) patients receiving immunosuppressive therapy including corticosteroids, and (i) organ and bone marrow transplant recipients. A single revaccination should be given if 5 years or more have passed since the first dose in subjects older than 10 years. In subjects 10 years of age and younger, revaccination should be given 3 years after the previous dose.
PPSV23 induces type-specific antibodies (T-cell–independent mechanisms) with a twofold rise within 2 to 3 weeks in 80% of young healthy adults. No correlation of antibody levels and protection has been determined. Antibody levels to these strains remain elevated for at least 5 years. In certain individuals, these levels decline within 10 years. Children may be protected for only 3 to 5 years. Elderly individuals and patients with chronic disease may have lower antibody levels produced with the vaccine. Children younger than 2 years do not respond adequately to the vaccine.
A number of other groups, including immunocompromised patients (e.g., leukemia, lymphoma, and multiple myeloma), dialysis patients, and patients with acquired immune deficiency syndrome, have reduced antibody production with the vaccine. Asymptomatic HIV-infected patients respond sufficiently to the vaccine. Patients with Hodgkin’s disease respond to the vaccine better before splenectomy, chemotherapy, or radiation therapy.
PPSV23 vaccine efficacy has been debated in the literature. Study results generally point to a reduction in invasive pneumococcal disease in the general population and in the elderly. In immunosuppressed populations, the reduction in invasive disease is estimated at 50% to 80% with immunization.37 Adults hospitalized with community-acquired pneumonia are significantly less likely to die if they have been immunized. In addition, immunized patients were less likely to have respiratory failure and had hospitalization stays that were shorter by 2 days.38
PPSV23 safety is well documented. Local reactions occur frequently within the first 48 hours and generally are mild. Local erythema and induration (30%), local discomfort (40%), and local swelling (3%) are the side effects observed most commonly. Revaccination has been associated with self-limited injection-site reactions more commonly than after the first dose. Severe systemic reactions occur rarely and consist of weakness, myalgia, headache, photophobia, chills, and fever.
Pneumococcal Conjugate Vaccine
Invasive pneumococcal disease occurs even more frequently in children younger than 2 years than in those older than 65 years. The infection ranges goes from nasopharyngeal carriage to bacteremia and meningitis. Because of the lack of immune responsiveness in children younger than 2 years when exposed to polysaccharide vaccines, a conjugate vaccine was developed to protect young children from certain strains of S. pneumoniae. However, the 13-valent vaccine is also licensed for individuals aged 50 years and older. The 13 valent vaccine (Prevnar-13) contains the conjugated capsular polysaccharides of serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. In clinical use, the vaccine is associated with a dramatic decline in invasive disease not only in immunized young children but also in individuals in all age groups.39
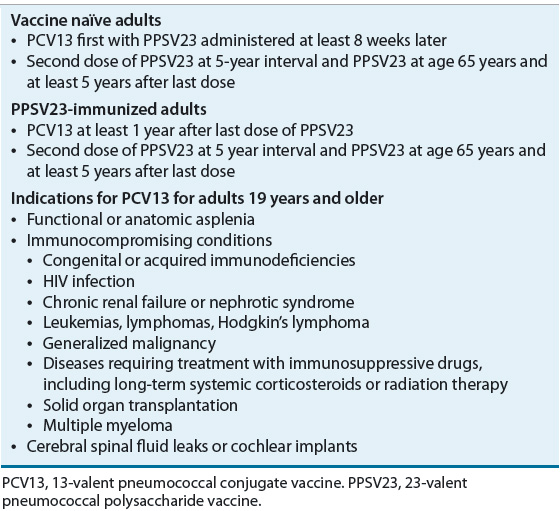
Immunization of Children PCV13 is administered as a 0.5-mL IM injection at 2, 4, and 6 months of age and between 12 and 15 months of age. A single dose of PCV13 should be administered to children aged 6 to 18 years with sickle cell disease or splenic dysfunction, HIV infection, immunocompromising conditions, cochlear implant, or cerebral spinal fluid leak should be immunized. PPSV23 can be used in conjunction with PCV13. PPSV23 should be administered after age 2 years and at least 2 months after the last dose of PCV13.16
Immunization of Adults The 13-valent pneumococcal conjugate vaccine (PCV13) offers some additional protection over pneumococcal polysaccharide vaccine (PPSV23) alone in this adult high-risk population. Several studies have shown protection that is at least as good as PPSV23. PCV13 is safe in these populations.40 Based on this information, the ACIP recommended PCV13 for adults with immunocompromising conditions40 (Table 102-3). PCV13 should be administered prior to PPSV23 in adults who have not been immunized previously. PCV13 should be administered with at least a year interval in those immunocompromised adults for whom it has been recommended and have already received one or more doses of PPSV23. No recommendations have been made for the use of PCV13 in other adult populations.
< div class='tao-gold-member'>