Fig. 4.1
Morphine structure. C3, C6, and the bridgehead nitrogen are the most common sites of linker (R1, R2, R3) attachment for the synthesis of haptens used in heroin/morphine vaccines. Immunogen structures are provided in Table 4.2
One such heroin vaccine was studied in a single monkey that had been trained to self-administer either heroin or cocaine (Bonese et al. 1974). (Note: Most vaccines intended to target either morphine or heroin have used haptens derived from modifications of morphine because of its greater stability in solution compared to heroin. The antibodies produced by such vaccines generally recognize or cross-react with and bind both morphine and heroin with high affinity. Because heroin is of greater interest as a target for addiction therapy, we will use the term heroin vaccines in this chapter for those derived from either heroin or morphine.) Subsequent vaccination against heroin reduced heroin, but not cocaine, self-administration. Similar results were obtained with the passive administration of heroin-specific antibodies (Killian et al. 1978). The attenuation of heroin self-administration by vaccination was substantial but could be overcome by greatly increasing the heroin unit dose. Because of this potential limitation, as well as increasing interest in methadone or naltrexone as treatment options, further efforts to develop heroin vaccines were not pursued at the time. It was not until nearly 25 years later that interest in opioid vaccines was rekindled by (1) the limited utilization of methadone and naltrexone, suggesting the need for additional types of pharmacotherapies; (2) an alarming increase in prescription opioid abuse and addiction (Dart et al. 2015); and (3) advances in addiction vaccine design owing to efforts with nicotine, cocaine, and infectious disease vaccines (Koff et al. 2013; Pentel et al. 2009).
4.1.4 Challenges in Developing Opioid Vaccines
The challenges posed by developing effective opioid vaccines are in part common to all addiction vaccines, and in part unique to opioid vaccines. The dominant shared challenge is reliably producing high concentrations of antibody in serum. This is important because the molar doses of these abused drugs are large and can easily exceed the binding capacity of serum antibody generated by vaccination (Table 4.1). Drug-specific antibody levels of up to 500 ug/ml, occasionally higher, can be elicited in rodents with nicotine (Cornish et al. 2013), cocaine (Kantak et al. 2000), or heroin (Kosten et al. 2014a; Anton and Leff 2006; Raleigh et al. 2013) vaccines. However, antibody levels achieved in clinical trials of nicotine or cocaine vaccines have been an order of magnitude or more lower, only rarely exceeding 100 ug/ml (Hatsukami et al. 2011; Martell et al. 2009; Esterlis et al. 2013). Vaccination of rodents generates higher antibody levels in part because higher immunogen doses can be administered. In humans, the largest tolerable volume of the injected solution is lower, and, in the case of alum adjuvant, the maximum allowable adjuvant dose is lower (because of concerns about aluminum toxicity) so that the maximum adsorbed dose of immunogen is also lower. For example, preclinical studies of the nicotine vaccine NicVAX (3′-aminonicotine conjugated to recombinant Pseudomonas endotoxin A) in rats used immunogen doses of up to 300 ug/kg (Cornish et al. 2013), while the highest dose used in clinical trials was approximately 6 ug/kg (Hatsukami et al. 2011). However, it is not clear whether the higher immunogen doses used in rodents are also needed in humans. Some animal studies have used adjuvants or dosing routes that are highly immunogenic but not appropriate or approved for humans, such as Freund’s adjuvant administered intraperitoneally. In both animals and humans, there is also considerable individual variability in antibody levels.
Table 4.1
Estimated single and daily doses of commonly abused drugs. The ratio of drug dose to antibody-binding capacity is calculated using a theoretical drug-specific antibody concentration of 200 μg/ml in serum
Drug | Dose (mg/kg) | Molar ratio of drug to antibody-binding capacity |
|---|---|---|
Nicotine | ||
Single dose | 0.015 | 0.5 |
Daily dose | 0.3 | 10 |
Cocaine | ||
Single dose | 0.5 | 9 |
Daily dose | 10 | 160 |
Methamphetamine | ||
Single dose | 1 | 40 |
Daily dose | 5 | 200 |
Heroin | ||
Single dose | 3 | 40 |
Daily dose | 9 | 120 |
Oxycodone | ||
Single dose | 1 | 18 |
Daily dose | 6 | 100 |
Challenges specific to opioid vaccines are that (1) most opioids have one or more active metabolites; (2) many different opioids are abusable, and opioid abusers often switch among opioids when their drug of choice is not available. It has not so far been possible to design a single immunogen that elicits antibodies which bind all of these opioids and metabolites; (3) some opioids have legitimate medical uses (analgesia, treatment of opioid addiction), and it is desirable for this option to be preserved; and (4) opioids are immunosuppressive and might impair an individual’s vaccine response.
Active metabolites
Heroin is a prodrug that is rapidly and sequentially converted in serum to its active metabolites 6-acetylmorphine (6-AM), morphine, and morphine-6-glucuronide (Fig. 4.2) (Rook et al. 2006a). Heroin is considered a prodrug because of its extremely rapid conversion to 6-AM (half-life 7 min in humans) resulting in very low serum and brain levels (Rook et al. 2006b) and because its affinity for the mu opioid receptor is substantially lower than that of its active metabolites (Inturrisi et al. 1983). Heroin is more lipophilic than these metabolites, enters the brain more readily, and may undergo some conversion to 6-AM within the brain, but the quantitative contribution of this pathway to heroin action is unclear (Andersen et al. 2009). Heroin’s immediate rewarding effects, those occurring within the first few minutes of a heroin dose, are probably attributable in greatest measure to 6-AM (Raleigh et al. 2013; Bogen et al. 2014). Conversion of 6-AM to morphine in serum is somewhat slower (half-life 22 min) but still rapid enough that morphine may contribute to the early behavioral effects of heroin (Rook et al. 2006b).
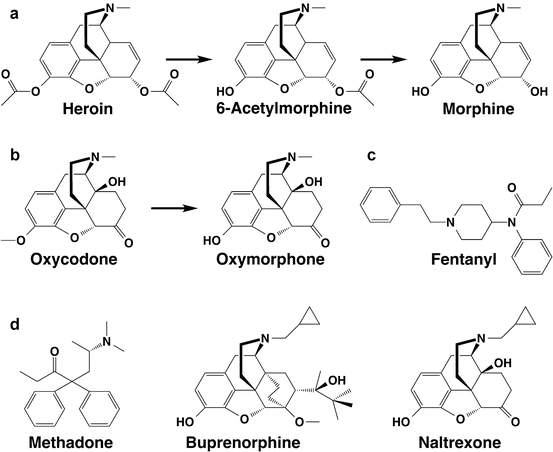

Fig. 4.2
Commonly abused opioids and their active metabolites. (a) Heroin and active metabolites. The downstream metabolite morphine-6-glucuronide, which is not formed in mice or rats, is not shown. (b) Oxycodone and its metabolite oxymorphone. Hydrocodone and hydromorphone (not shown) have analogous structures but lack a C14 hydroxyl group. (c) Fentanyl. (d) Nontargeted opioids: methadone, buprenorphine, and naltrexone
It is not entirely clear to what extent vaccines targeting heroin owe their efficacy to binding heroin per se, its active metabolites, or both (Fig. 4.3). Even though heroin is a prodrug, the binding of heroin by antibody could potentially slow or block its conversion to 6-AM and downstream metabolites. However, this is unlikely because immunization of rats with vaccines that bind and retain substantial heroin in serum does not prevent the rapid accumulation of 6-AM in serum, within minutes of a heroin dose. One such vaccine was shown to reduce 6-AM but not heroin distribution to the brain in rats after a single heroin dose, yet it markedly attenuated heroin analgesia and self-administration (Raleigh et al. 2013). Additional data argue against the binding of morphine being important, at least for blocking the very early effects of heroin; a “dynamic” heroin vaccine (see Sect. 4.2), which selectively retains heroin and 6-AM but not morphine in serum, is effective in blocking heroin self-administration (Schlosburg et al. 2013). Together, these studies implicate 6-AM in serum as the primary vaccine target for altering the early effects of heroin. The role of morphine-6-glucuronide (M-6-G) has not been studied in the context of vaccines because rodents do not produce this metabolite (Antonilli et al. 2005), but morphine-specific antibodies have been shown to bind M-6-G in vitro (Anton and Leff 2006; Raleigh et al. 2013; Kosten et al. 2013).
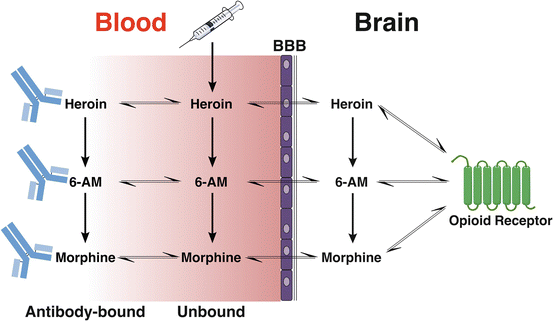

Fig. 4.3
Possible fates of heroin and active metabolites in the blood and brain in the presence of morphine-specific antibodies. Heroin administered to a vaccinated individual may undergo (1) distribution directly into the brain, (2) conversion to its more active metabolites, or (3) binding to antibody. 6-AM and morphine share these outcomes. Antibody generated by heroin vaccines can bind each of these compounds in serum and potentially slow or reduce their entry into the brain. It is also possible that the binding of heroin to antibody slows its conversion to the more active 6-AM, but initial data in rats suggests this is not a major contributor to vaccine efficacy
Oxycodone and hydrocodone, the most commonly abused prescription opioids, also have active metabolites (Fig. 4.2). Fortunately, antibodies directed at oxycodone or hydrocodone also bind these metabolites with comparable affinities (Pravetoni et al. 2012a, 2013).
Many abusable opioids: the specificity of opioid vaccines
The abuse of prescription opioids such as oxycodone and hydrocodone increased dramatically in the first decade of this century and rose to more than ten times the prevalence of heroin abuse in the USA (Dart et al. 2015). Those abusing prescription opioids may switch among them depending on availability and cost, or transition to heroin. Addressing this complex epidemiology may require vaccines that can target each of the most commonly abused opioids.
It does not appear feasible to design a single immunogen that will elicit antibodies that bind all of the commonly abused opioids. The commonly abused opioids, as well as the most commonly used therapeutic opioids, differ sufficiently in structure that antibodies generally do not appreciably cross-react among them (Fig. 4.2). For example, antibodies generated using haptens based on morphine generally bind heroin and its active metabolites avidly but have orders of magnitude lower affinities for oxycodone or hydrocodone (Pravetoni et al. 2012b; Stowe et al. 2011b). Targeting a wide range of abusable opioids will require a different approach such as coadministering heroin and oxycodone vaccines, as discussed below (Sect. 4.6).
The prescription opioid fentanyl is less commonly abused. Antibodies elicited by heroin or oxycodone vaccines do not appreciably bind fentanyl (Raleigh et al. 2013; Pravetoni et al. 2013), but fentanyl-based haptens can generate such antibodies and block fentanyl analgesia in rodents (Torten et al. 1975).
There is a beneficial aspect to the selectivity of antibodies elicited by heroin or oxycodone vaccines in that their much lower affinities for the addiction treatment medications methadone, buprenorphine, and naltrexone should allow these medications to retain their clinical efficacy for treating opioid addiction. It should be possible, in principle, to combine opioid vaccines with these opioid addiction treatment medications without compromising the efficacy of either. The role of vaccines in this setting could be to (1) enhance blockade of the effects of heroin or prescription opioids that are abused despite maintenance on treatment medications, (2) provide blockade of abused opioid effects during periods of noncompliance with treatment medications, or (3) provide blockade of opioid effects when patients transition off their treatment medications.
4.2 Heroin Vaccine Design
4.2.1 Opioid Scaffold and Linker Position
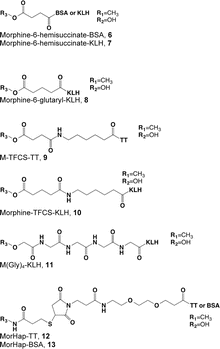
Most opioid haptens use C6 or the bridgehead (piperidinyl) nitrogen for linker attachment (Fig. 4.1, Table 4.2). Two of the earliest reports described haptens derived from morphine with linkers at C3 that elicited antibodies with activity against both morphine and heroin. The more extensively studied of these was later reported to have been analyzed incorrectly and to actually have the linker at C6 (Wainer et al. 1972a, b). Findlay et al. (1981) analyzed a range of opioid haptens based on morphine or oxycodone and concluded that haptens using the C6 linker position showed greatest recognition of structural differences around the bridgehead nitrogen and C14, while haptens using the bridgehead nitrogen linker position showed greatest recognition of structural differences at C3 or C6. This is consistent with the general principle that structural recognition by antibodies is greatest for aspects of a hapten distant from the site of linker attachment, because linker attachment hides the linker position and its immediate environment from presentation to B-cell receptors (Matyas et al. 2014).
Table 4.2
Heroin immunogen structures and specificities determined by ELISA
R1: bridgehead nitrogen | Opioid | Relative specificity (%) | Reference |
|---|---|---|---|
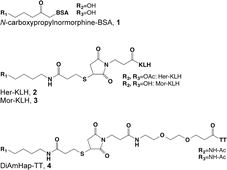 | 1. Morphine Normorphine Hydromorphone Dihydromorphine | 100 90 79 34 | Findlay et al. (1981) |
2. Morphine Heroin 6-AM | 100 267 32,000 | ||
3. Morphine Heroin 6-AM | 100 8.5 <1.2 | ||
4. Morphine Heroin 6-AM | 100 1033 425 | Matyas et al. (2014) | |
R2: C3 | |||
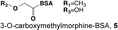 | 5. Morphine Heroin Normorphine | 100 133 100 (relative to dihydromorphone) | |
R3: C6 | |||
 | 6. Morphine Heroin Codeine Hydromorphone | 100 110 70 22 | |
7. Morphine 6-AM | 100 173 | ||
8. Morphine Heroin | 100 0.46 | Li et al. (2011) | |
9. Morphine Heroin 6-AM | Described as “equivalent specificity” | Anton and Leff (2006) | |
10. Morphine Heroin 6-AM | 100 12 40 | Li et al. (2014) | |
11. Morphine Heroin 6-AM M-6-G | 100 167 125 50 | ||
12. Morphine Heroin 6-AM | 100 <3.6 54 | ||
Heroin vaccines
Heroin vaccines with C6 linkers are generally synthesized from morphine. Although these lack both the 3- and 6-acetyl groups present on heroin, the elicited antibodies bind heroin, 6-AM and morphine with comparable affinities, consistent with the notion that these positions are not a critical component of the hapten face interacting with antibody (Anton and Leff 2006; Raleigh et al. 2013; Kosten et al. 2013). However, alterations at C8 or C14 are recognized so that these antibodies have little affinity for oxycodone or hydrocodone (Findlay et al. 1981).
Several reports describe heroin vaccines using the bridgehead nitrogen linker position (Schlosburg et al. 2013; Findlay et al. 1981; Matyas et al. 2014). A “dynamic” hapten placed a linker at this position on heroin, rather than morphine, to retain the acetyl groups at C3 and C6 and allow the hapten to be converted in vivo sequentially from the diacetyl to the monoacetyl and then the non-acetylated analogs (Stowe et al. 2011b). This sequence was intended to mimic in vivo heroin metabolism and expose the vaccinated animal to immunogen analogs of each of the active heroin metabolites. Although the rapid deacetylation of this hapten in vivo precluded measuring the presumed conversion, the antibodies produced by vaccination bound 6-AM with higher affinity than heroin or morphine, and blocked heroin analgesia better than the analogous hapten with OH groups at C3 and C6. An additional study of the bridgehead nitrogen linker position using stable substituents on C3 and/or C6, so that sequential exposure to acetylated analogs was not a feature, showed that this immunogen structure was also capable of generating antibodies against heroin, 6-AM, and morphine (Matyas et al. 2014). It appears, therefore, that a variety of approaches involving either C6 or the bridgehead nitrogen are available to generate antibodies of interest for therapeutic applications. These approaches have not been extensively compared so it is unclear which, if any, is best.
4.2.2 Linker Structure
A surprisingly wide variety of linkers have been used for heroin or oxycodone vaccines and found to be satisfactory. Linker length has varied from 2 to 24 atoms (Table 4.2). In general, linkers have been designed to be flexible and to have low structural complexity. This reflects an attempt to make the linker itself less immunogenic, so that the immune response is focused more on the hapten.
It is not apparent that any one linker length or structure is superior to all others, and this is a challenging question to address. Linker length and composition may affect not just the ability of a hapten to engage B-cell receptors, they may also affect the density of hapten that can be attached to its carrier protein, which is known to be a critical determinant of immunogenicity (Carroll et al. 2011). Hapten density on its carrier (the haptenation ratio) is often not measured or, in the case of very large protein carriers such as KLH, is difficult to measure accurately. As a result, the influence of the linker on haptenation chemistry is difficult to separate from its influence on the ability of immunogen to engage B-cell receptors. In addition, reported heroin vaccines have used many different carrier proteins and adjuvants, and their efficacy has been analyzed using different in vitro or in vivo assays, further confounding the ability to compare vaccines across or even within studies.
4.2.3 Carrier and Hapten Density
Opioid vaccines have used many of the same carrier proteins known to be most effective for other types of haptens including nicotine and cocaine (Table 4.2). These include bovine serum albumin (BSA, not suitable for human use because of dietary exposure to this protein), tetanus toxoid, and keyhole limpet hemocyanin (KLH). As with linkers, direct comparisons are few but tetanus toxoid and KLH were found equally effective for an oxycodone vaccine. When measured (using BSA conjugates as models), molar ratios of 15–22 haptens/protein have been effective (Pravetoni et al. 2012b; Stowe et al. 2011b; Matyas et al. 2014), and more so than lower ratios (unpublished data). More extensive experience with other hapten-protein conjugate vaccines suggests that optimization of carrier haptenation is a critical aspect of conjugate vaccine design, regardless of the carrier (Carroll et al. 2011).
4.2.4 Adjuvant
Adjuvants used in opioid vaccines reflect those used for other types of immunizations. The most common is alum because of its acceptability for human use and long track record of efficacy and safety. Other adjuvants used clinically or experimentally, for unrelated vaccines, have unsurprisingly also shown efficacy with opioid vaccines. A combination of alum and CpG was more effective than either one alone for a heroin vaccine (Bremer et al. 2014), consistent with a previous report with a nicotine vaccine (McCluskie et al. 2013). Monophosphoryl lipid A (MPLA) embedded in a liposome, mixed with a heroin hapten-tetanus toxoid conjugate so that the MPLA liposome functioned as an adjuvant, elicited high titers of opioid antibodies (Matyas et al. 2013). Surprisingly little is known about the extent to which adjuvant potencies in rodents or even primates are predictive of results in humans, and this question will likely require clinical trials to identify the best options.
4.3 Heroin Vaccine Immunogenicity
4.3.1 Affinity and Specificity
The amount of drug that can be bound by antibody is a function of both the amount of antibody available and the affinity, or strength of binding, between antibody and drug. Opioid antibody affinity has been measured for morphine rather than heroin due to heroin’s instability in serum. Using equilibrium dialysis or radioimmunoassay, Kd values of 24.5, 16.6 (Stowe et al. 2011b), and 2.5–4.0 (Anton and Leff 2006) were reported for three vaccines (immunogens 2, 3, and 9 of Table 4.2). These values compare favorably with Kd’s measured for nicotine or cocaine vaccines. Competition ELISA methods, which are simpler and less expensive, can be used in lieu of equilibrium dialysis or radioimmunoassay to compare relative affinities and specificities of various ligands for antibody but cannot generate accurate Kd values for free drug (see following paragraph).
4.3.2 Antibody Concentration
Serum antibody titers measured by ELISA are often used as a surrogate for concentration, because their measurement is quick and inexpensive. However, ELISA titers actually measure antibody binding to the coating antigen, which includes linker and carrier protein, rather than to free drug alone. ELISA titers are also sensitive to assay conditions, making comparisons between laboratories uninformative. ELISA assays can be calibrated to provide an estimate of serum antibody concentration using a monoclonal opioid antibody (Raleigh et al. 2013) or nonspecific IgG (Kosten et al. 2013). Concentrations can also be obtained by equilibrium dialysis or radioimmunoassay, which avoid the potential confounding effects of linker or carrier protein.
Estimates of serum anti-morphine antibody concentrations obtained by equilibrium dialysis or a suitably calibrated ELISA range from 200 to 800 ug/ml, (Anton and Leff 2006; Raleigh et al. 2013; Kosten et al. 2013; Stowe et al. 2011b) similar to those reported with other addiction vaccines (although one very high value of 2840 ug/ml was also reported (Stowe et al. 2011b). This range can be verified by measuring the concentrations of opioids retained in serum in vaccinated animals, to provide an estimate of the minimum serum antibody concentration that would have to be present to bind them. This calculation gives similar estimates, e.g., 300–400 ug/ml in rats receiving heroin or 6-AM (unpublished calculation based on (Raleigh et al. 2014)). To put these serum antibody concentrations in perspective, most infectious disease vaccines produce serum neutralizing antibody concentrations that are several orders of magnitudes lower (Scott et al. 2011). It can be argued that efforts to optimize the immunogenicity of heroin vaccines have already been quite successful and that the limitations of vaccine efficacy stem from the difficulty of the task: the need to bind the large heroin doses used by opioid addicts.
4.3.3 Vaccination During Opioid Administration
It is likely that opioid abusers will need to be vaccinated before they become abstinent from using opioids, so that high antibody levels are present when abstinence is attempted. In animals, concurrent exposure to drugs such as nicotine or methamphetamine during vaccination does not impair the immune response to the corresponding addiction vaccines (Hieda et al. 2000; Byrnes-Blake et al. 2001). Similarly, in rats vaccinated against heroin, subsequent exposure to heroin does not impair their antibody response to booster doses of vaccine (Raleigh et al. 2014). These data suggest that vaccination against heroin will not be impaired by concurrent heroin use.
An additional issue is that morphine is known to be immunosuppressive, and heroin addicts have been reported to have a lower antibody response to hepatitis B vaccines than controls (Rodrigo et al. 1992). Many heroin addicts also have chronic infections such as HIV or hepatitis C that can impair immune responses (Quaglio et al. 2002). The opioid-abusing population may require different or more aggressive vaccination regimens to achieve the highest possible antibody levels from opioid vaccines.
4.4 Heroin Vaccine Efficacy
4.4.1 Opioid Doses
Clinical laboratory investigations show rewarding effects of heroin in addicts at doses as low as 0.4 mg/kg administered i.v. (Comer et al. 2008), but abused doses are generally much larger. Studies of heroin maintenance as a therapeutic option, which allow heroin addicts to adjust their dose, report mean daily doses of 500–600 mg (7–9 mg/kg) typically injected i.v. in 1–3 doses (Reuter 2009; Haemmig and Tschacher 2001; Rentsch et al. 2001). Doses used likely vary depending upon heroin price and purity and degree of opioid tolerance. Thus, the target dose that must be addressed by a vaccine is unclear but by any estimate it is considerable.
Animal models of heroin abuse often differ from the doses or manner in which addicts use these drugs (Table 4.3). Heroin or morphine self-administration protocols typically use relatively low i.v. unit doses, e.g., 0.06 mg/kg/infusion although they may achieve a substantial total dose, up to 2 mg/kg, over a 1–2 h session (Bonese et al. 1974; Schlosburg et al. 2013; Raleigh et al. 2014). Higher heroin or morphine doses are used to study vaccine effects on opioid analgesia or locomotor activity, but these are usually administered s.c. and are absorbed more slowly than with i.v. dosing. These differences between dosing regimens in animals and humans add to the difficulty of predicting the clinical impact of opioid vaccines.
Table 4.3
Effects of heroin/morphine vaccines on opioid addiction-relevant behaviors in rats
Immunogen | Behavior attenuated/blocked | Opioid | Dose and route | Reference |
|---|---|---|---|---|
Her-KLH, 2 | CPP Hot-plate von Frey acquisition reacquisition reinstatement | Morphine Heroin | 4 mg/kg, s.c. 0.4 mg/kg, s.c. 1 mg/kg, s.c. 1 mg/kg, s.c. 0.06 mg/kg/inf, i.v. 0.06 mg/kg/inf, i.v. up to 6.5 mg/kg/6-h 0.18 mg/kg, i.v. | |
DiAmHap-TT, 4 MorHap-TT, 12
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|