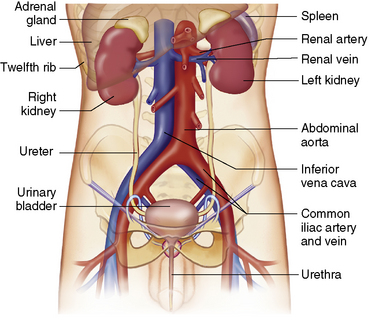
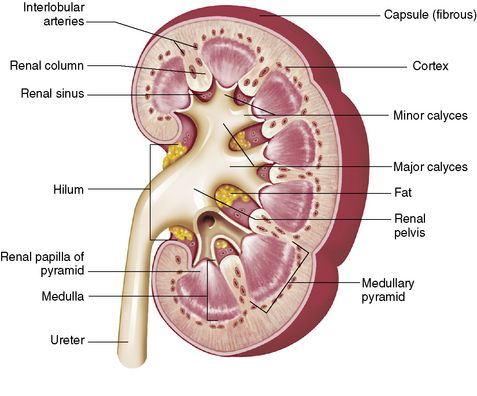
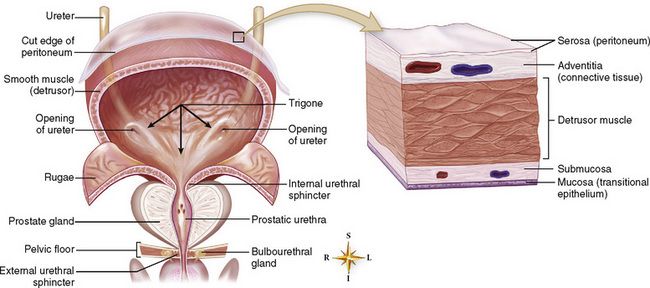
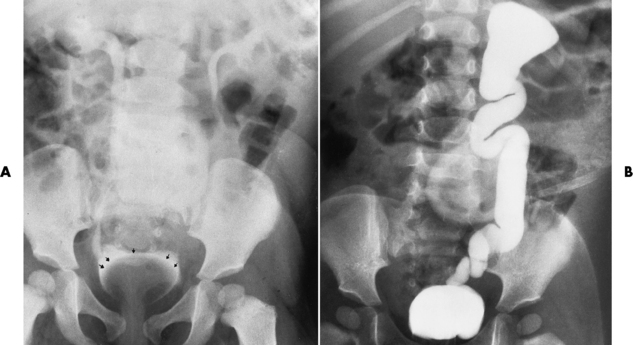
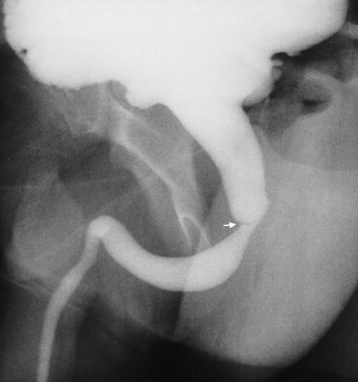
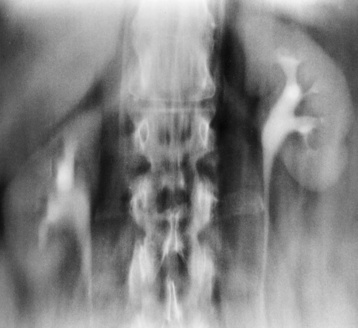
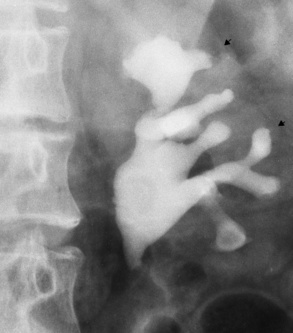
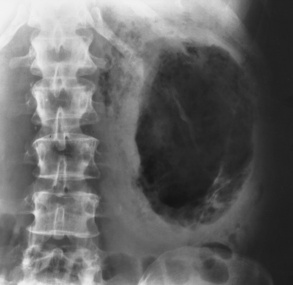
Chapter 6 After reading this chapter, the reader will be able to: 1 Classify the more common diseases in terms of their attenuation of x-rays 2 Explain the changes in technical factors required for obtaining optimal-quality radiographs in patients with various underlying pathologic conditions 3 Define and describe all bold-faced terms in this chapter 4 Describe the physiology of the urinary system 5 Identify anatomic structures on both diagrams and radiographs of the urinary system 6 Differentiate the various pathologic conditions affecting the urinary system and their radiographic manifestations The urinary system consists of the kidneys, ureters, and bladder (Figure 6-1). The functional unit of the kidney is the nephron. Each kidney contains more than a million nephrons, which filter waste products from the blood, reabsorb water and nutrients (e.g., glucose and amino acids) from the tubular fluid, and secrete excess substances in the form of urine. In an average person, the nephron filters about 190 L of water out of glomerular blood each day. This enormous amount is many times the total volume of blood in the body. However, only a small proportion of this water (1 to 2 L) is excreted in the urine. Therefore, more than 99% of water is reabsorbed into tubular blood. The formation of urine begins in the glomerulus, a tuft of capillaries with very thin walls and a large surface area. The blood pressure within the glomerulus is higher than that in Bowman’s capsule, which surrounds it. This difference causes the filtration of fluid into Bowman’s capsule that is equivalent to plasma containing neither protein nor red blood cells (if the nephron is healthy). The initial urine proceeds into the proximal convoluted tubule, where a large amount of water and virtually all nutrients are reabsorbed into the blood capillaries surrounding the tubules. The amount of sodium and chloride reabsorbed is determined by the concentration of these substances in the body, and it occurs at a variable rate designed to keep the osmotic pressure of the body constant. This process is greatly influenced by two hormones: antidiuretic hormone (ADH), secreted by the posterior pituitary gland, and aldosterone, secreted by the adrenal glands. After passing through the proximal tubule, the fluid flows through the loop of Henle, a complex structure consisting of a descending limb, a loop, and an ascending limb. Following the reabsorption of salt and water in the loop of Henle, the distal convoluted tubules permit the excretion of concentrated urine by actively secreting substances such as potassium ions (K+), hydrogen ions (H+), and some drugs. In this way the kidney plays an essential role in maintaining salt or electrolyte balance and acid-base balance of blood and body fluids. Eventually, urine passes from the collecting tubules, whose openings are in the papillae, into the calyces, and on to the funnel-shaped renal pelvis and tubular ureters (Figure 6-2). Peristaltic waves (about 1 to 5 per minute) force the urine down the ureters and into the bladder. The ureters enter the bladder through an oblique tunnel that functions as a valve to prevent backflow of urine into the ureters (vesicoureteral reflux) during bladder contraction. The bladder acts as a reservoir for the urine before it leaves the body (Figure 6-3). The openings of the two ureters lie at the posterior corners of the triangle-shaped floor (the trigone), and the urethral opening is situated at the anterior lower corner. Filling of the bladder (about 250 mL in the average person) stimulates autonomic nerve endings in the wall that are perceived as a distended sensation and the desire to void (micturate). A complicated sequence of bladder contractions and relaxation of the sphincter muscles permits the bladder to expel urine from the body through the urethra. Voluntary contraction of the external sphincter to prevent or terminate micturition is learned and is possible only if the motor system is intact. Nervous system injury (cerebral hemorrhage, spinal cord injury) results in involuntary emptying of the bladder at intervals (incontinence). Unilateral renal agenesis (solitary kidney) is a rare anomaly that may be associated with a variety of other congenital malformations (Figure 6-4). Before the diagnosis can be made, it is essential to exclude a nonfunctioning, diseased kidney and prior nephrectomy. Unilateral renal agenesis results from a failure of the embryonic renal bud or renal vascular system to form. In true renal agenesis, the ureter and corresponding half of the trigone are missing also. Ultrasound (ultrasonography) or computed tomography (CT) can demonstrate the absence of renal tissue. A solitary kidney tends to be larger than expected, reflecting compensatory hypertrophy. A supernumerary kidney is also a rare anomaly. The third kidney is usually small and rudimentary and possesses a separate pelvis, ureter, and blood supply. Although supernumerary kidneys function normally, they tend to cause secondary infections that eventually may require their removal. A small, hypoplastic kidney often appears as a miniature replica of a normal kidney, with good function and a normal relationship between the amount of parenchyma and the size of the collecting system (Figure 6-5). Renal hypoplasia must be differentiated from an acquired atrophic kidney, which is small and contracted because of vascular or inflammatory disease that has reduced the volume of renal parenchyma. Compensatory hypertrophy is an acquired condition that develops when one kidney is forced to perform the function normally carried out by two kidneys (see Figure 6-5). This phenomenon may follow unilateral renal agenesis, hypoplasia, atrophy, or nephrectomy. The ability of the kidney to undergo compensatory hypertrophy is greatest in children and diminishes in adulthood. Ultrasound demonstrates the size of the renal parenchyma, calyces, and pelvis without the use of a contrast agent or ionizing radiation to provide a diagnosis. Malrotation of one or both kidneys may produce a bizarre appearance of the renal parenchyma, calyces, and pelvis that suggests a pathologic condition when in reality the kidney is otherwise entirely normal (Figure 6-6). Abnormally positioned kidneys (ectopic kidney) may be found in various locations, from the true pelvis (pelvic kidney) (Figure 6-7) to above the diaphragm (intrathoracic kidney) (Figure 6-8). Pelvic kidneys occur much more frequently than intrathoracic kidneys. Whenever only one kidney is seen on intravenous urography, a full view of the abdomen is essential to search for an ectopic kidney. Although the ectopic kidney usually functions, the nephrogram and the pelvicalyceal system may be obscured by overlying bone and fecal contents. Patient history can distinguish a true pelvic kidney from a kidney transplant, which typically is located in the right pelvis. Crossed ectopia refers to a situation in which an ectopic kidney lies on the same side as the normal kidney and is very commonly fused with it. Horseshoe kidney is the most common type of fusion anomaly. In this condition, both kidneys are malrotated and their lower poles are joined by a band of normal renal parenchyma (isthmus) or connective tissue (Figure 6-9). The ureters arise from the kidneys anteriorly instead of medially, and the lower pole calyces point medially rather than laterally. The pelves are often large and flabby and may simulate obstruction. Obstruction at the ureteropelvic junction may occur because of the anterior position of the ureters. Complete fusion of the kidneys is a rare anomaly that produces a single irregular mass that has no resemblance to a renal structure. The resulting bizarre appearance has been given such varied names as disk, cake, lump, and doughnut kidney. Duplication (duplex kidney) is a common anomaly that may vary from a simple bifid pelvis (Figure 6-10) to a completely double pelvis ureter (Figure 6-11) and ureterovesical orifice. The ureter draining the upper renal segment enters the bladder below the ureter draining the lower renal segment. Complete duplication can be complicated by obstruction or by vesicoureteral reflux with infection. Vesicoureteral reflux and infection more commonly involve the ureter draining the lower renal segment; obstruction more frequently affects the upper pole, where it can cause a hydronephrotic mass that displaces and compresses the lower calyces. A ureterocele is a cystic dilatation of the distal ureter near its insertion into the bladder. In the simple (adult) type, the opening in the ureter is situated at or near the normal position in the bladder, usually with stenosis of the ureteral orifice and with varying degrees of dilatation of the proximal ureter. The stenosis leads to prolapse of the distal ureter into the bladder and dilatation of the lumen of the prolapsed segment. Ectopic ureteroceles are found almost exclusively in infants and children; most are associated with ureteral duplication. The appearance of a ureterocele on intravenous urography depends on whether opaque medium fills it. If it is filled, the lesion appears as a round or oval density surrounded by a thin radiolucent halo representing the wall of the prolapsed ureter and the mucosa of the bladder (cobra head sign) (Figure 6-12A). When the ureterocele is not filled with contrast material, it appears as a radiolucent mass within the opacified bladder in the region of the ureteral orifice (Figure 6-12B). Ultrasound is the modality of choice to evaluate infants and children. Children with ureteral duplication have an 80% incidence of an associated ureterocele. On ultrasound images, a ureterocele appears as a round cystlike structure within the bladder (Figure 6-12C). On intravenous urography, an ectopic ureterocele typically appears as a large, eccentric filling defect impressing the floor of the bladder (Figure 6-13). The ureterocele arises from the ureter draining the upper segment of the duplicated collecting system. A mass effect, representing hydronephrosis, often involves the upper pole of the kidney and causes downward and lateral displacement of the lower portion of the collecting system. Posterior urethral valves are thin, transverse membranes, found almost exclusively in males, that cause bladder outlet obstruction and may lead to severe hydronephrosis, hydroureter, and renal damage. The thin, transverse membranes work as a reverse valve, meaning that catheterization is normal but the valve prevents antegrade flow. They are best demonstrated on a voiding cystourethrogram (Figure 6-14). The proximal urethra is dilated and the thin, lucent, transverse membrane of the valve can be identified. Summary of Findings for Congenital/Hereditary Diseases IVU, Intravenous urography; US, ultrasound; VCUG, voiding cystourethrography. the glomeruli extremely permeable, allowing albumin and red blood cells to leak into the urine (resulting in proteinuria or hematuria). A decreased glomerular filtration rate results in oliguria, a smaller-than-normal amount of urine. The intravenous urographic findings in glomerulonephritis depend on the duration and severity of the disease process and on the level of renal function. In patients with acute glomerulonephritis, the kidneys may be normal or diffusely increased in size with smooth contours and normal calyces. A loss of renal substance in chronic glomerulonephritis produces bilateral small kidneys (Figure 6-15). The renal outline remains smooth and the collecting system is normal, unlike the irregular contours and blunted calyces seen in chronic pyelonephritis. Ultrasound may demonstrate these findings more efficiently without contrast enhancement or radiation exposure. The urographic hallmark of chronic pyelonephritis is patchy calyceal clubbing with overlying parenchymal scarring (Figure 6-16). Initially, there is blunting of the calyces, which then become rounded or clubbed. Fibrotic scarring causes a cortical depression overlying the dilated calyx. Progressive cortical atrophy and thinning may be so extensive that the tip of the blunted calyx appears to lie directly beneath the renal capsule. The urographic findings may be unilateral or bilateral and are often most pronounced at the poles. If calyceal changes are minimal, the overlying cortical depressions may simulate lobar infarctions or normal kidney lobulations. However, in chronic pyelonephritis, the cortical depression lies directly over a calyx rather than between calyces as in lobar infarctions or congenital lobulation. Chronic pyelonephritis may progress to end-stage renal disease with small, usually irregular, poorly functioning kidneys. Emphysematous pyelonephritis is a severe form of acute parenchymal and perirenal infection with gas-forming bacteria that occurs virtually only in diabetic patients and causes an acute necrosis of the entire kidney. The presence of radiolucent gas shadows within and around the kidney is pathognomonic of emphysematous pyelonephritis (Figure 6-17). CT is the preferred modality for localizing the gas patterns within and around (perinephric) the kidney. The affected region appears as mottled areas of low attenuation.
Urinary System
Physiology of the urinary system
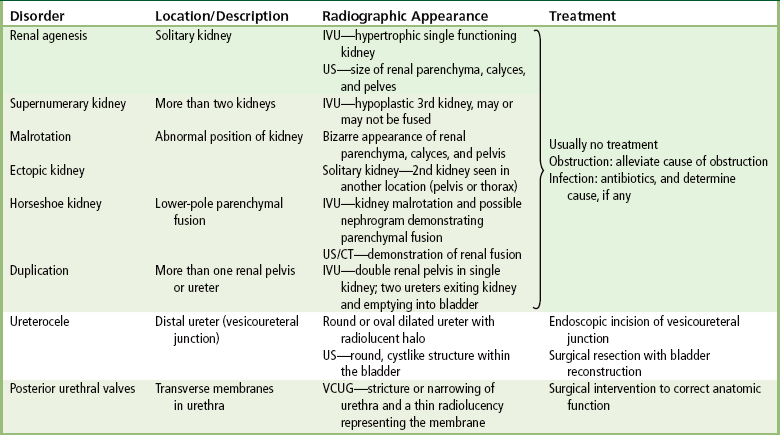
Congenital/hereditary diseases
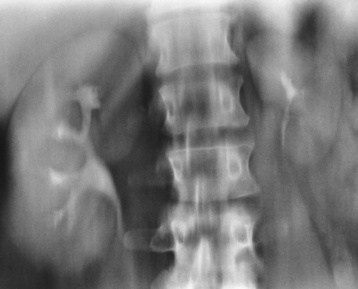
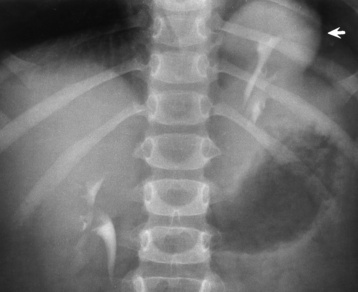
Anomalies of Rotation, Position, and Fusion
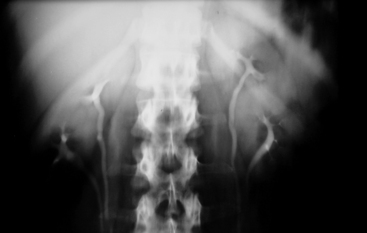
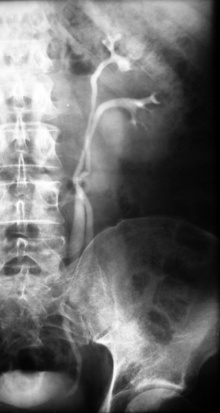
Anomalies of Renal Pelvis and Ureter
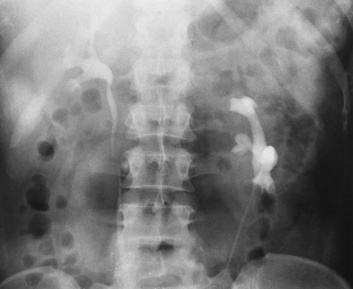
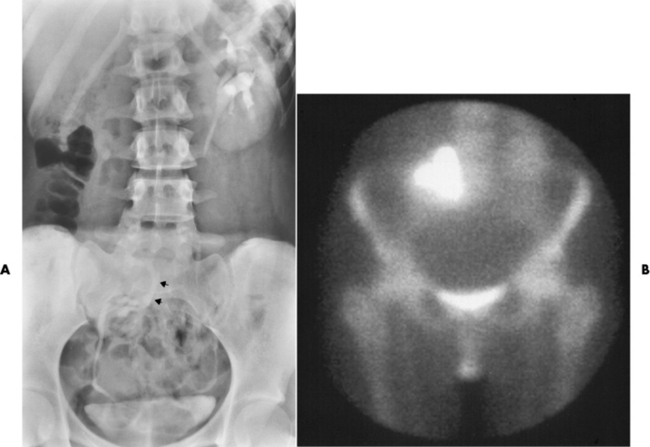
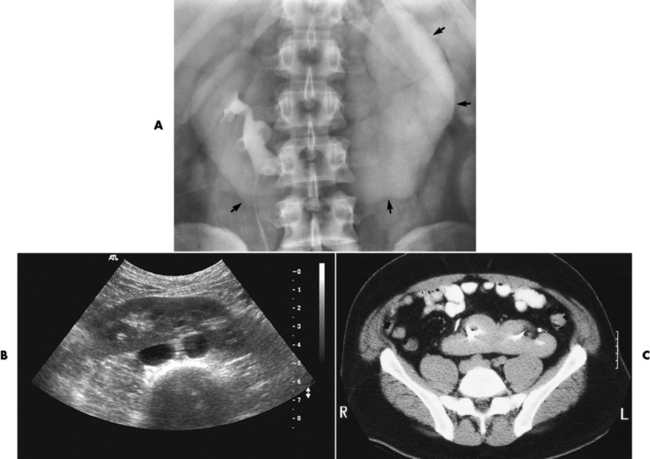
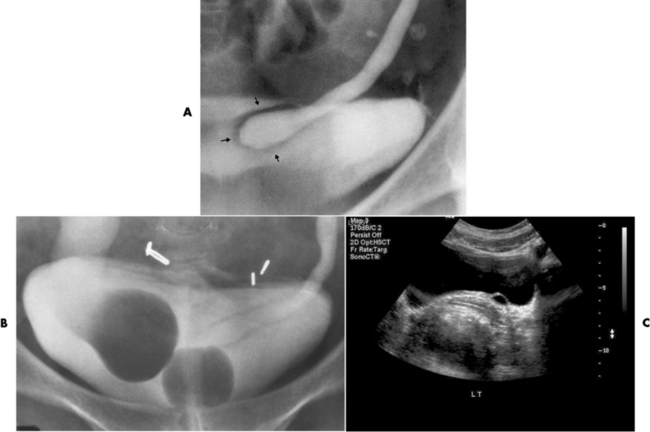
Ureterocele
Radiographic Appearance
Posterior Urethral Valves
Inflammatory disorders
Radiographic Appearance
Pyelonephritis
Radiographic Appearance
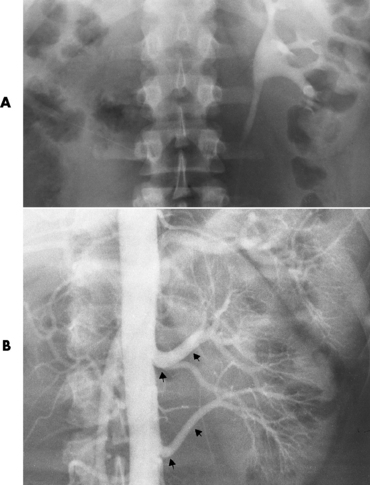
Emphysematous Pyelonephritis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Urinary System