16 • Filtration in the glomerulus of most small molecules from blood plasma to form an ultrafiltrate of plasma • Selective reabsorption in the tubule of most of the water and some other molecules from the ultrafiltrate, leaving behind excess and waste materials to be excreted • Secretion in the tubule of some excretory products directly from blood into the urine • Maintenance of the acid-base balance by selective secretion by the tubule of H+ ions into the urine The kidney also has hormonal and metabolic functions: • Renin, synthesised in the kidney, is a component of the renin-angiotensin-aldosterone mechanism that controls blood pressure. • Erythropoietin, synthesised in the kidney, stimulates the production of erythrocytes in the bone marrow and thus regulates the oxygen-carrying capacity of the blood. • Vitamin D, which regulates calcium balance, is converted to an active form in the kidney. FIG. 16.1 The urinary system FIG. 16.2 Kidney FIG. 16.3 Kidney FIG. 16.4 Basic organisation of the nephron, collecting system and renal vasculature (caption continues opposite) 2. The loop of Henle includes the distal straight part of the proximal tubule, the pars recta, the thin descending and ascending limbs and the thick ascending limb. The difference between these parts is due to differences in the epithelium. The thin segments of the loop of Henle dip down into the medulla where they form a hairpin bend. The length of the loop of Henle varies from short to long, depending on the location of the renal corpuscle of the particular nephron. The corpuscles of short-looped nephrons tend to be located in the superficial and midcortical regions, the loops extending very little beyond the corticomedullary junction. Long-looped nephrons are mainly associated with juxtamedullary corpuscles; a small proportion of long loops almost reach the tips of the renal papillae, but successively greater numbers turn back at higher levels as necessitated by the tapering shape of the medullary pyramids. The limbs of the loop of Henle are closely associated with parallel wide capillary loops, the vasa recta (not shown in this diagram), which arise from the efferent arterioles of glomeruli located near the corticomedullary junction. The vasa recta descend into the medulla then loop back on themselves to drain into veins at the junction of the medulla and cortex. The main function of the loops of Henle is to generate a high osmotic pressure in the extracellular fluid of the renal medulla; the mechanism by which this is achieved is known as the counter–current multiplier system (see Fig. 16.26). In some animals, the loop of Henle plays a major role in reabsorption of water from the glomerular filtrate back into the circulation via the vasa recta; however, this function is of lesser importance in the human kidney. The medulla can be divided into different zones according to the components of the loop of Henle that are present: the inner medulla contains only thin limbs of the loop of Henle, the inner stripe of the outer medulla contains thick descending limbs as well as thin limbs and the outer stripe of the outer medulla contains thick ascending limbs as well as thick descending limbs and thin limbs. 4. The collecting tubule is the straight terminal portion of the nephron, several collecting tubules converging to form a collecting duct. The collecting ducts descend through the cortex in parallel bundles called medullary rays (see Fig. 16.5), progressively merging in the medulla to form the large ducts of Bellini, which open at the tips of the renal papillae to discharge urine into the pelvicalyceal system. The collecting tubules and ducts are not normally permeable to water. However, in the presence of antidiuretic hormone (ADH) secreted by the posterior pituitary, the collecting tubules and ducts become permeable to water. Thus the high osmotic pressure generated by the counter-current multiplier system into the interstitial tissues of the medulla removes water that is returned to the general circulation via the vasa recta. The loops of Henle and ADH thus provide a mechanism for the production of urine that is hypertonic with respect to plasma. Renal vasculature FIG. 16.5 Kidney, monkey FIG. 16.6 Renal cortex FIG. 16.7 Development of the renal corpuscle FIG. 16.8 Renal corpuscle FIG. 16.9 Glomerulonephritis
Urinary system
Introduction
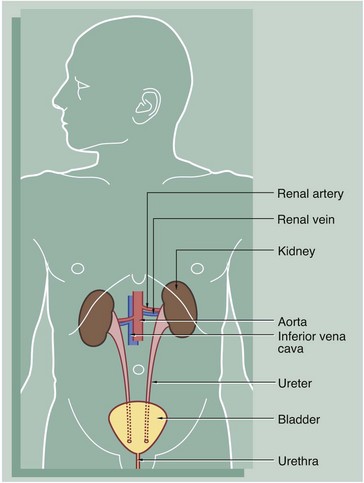
The urinary system comprises two kidneys, two ureters, a bladder and a urethra. Urine is produced in the kidneys and flows down the ureters to the bladder where it is stored until voided via the urethra. No further modification of the urine takes place after it leaves the kidneys. The kidneys and ureters are found in the retroperitoneum, while the urinary bladder is in the anterior part of the pelvis.
Blood is supplied to each kidney by the renal arteries, which arise from the aorta. One or more renal veins drains the blood from each kidney to the inferior vena cava. The total blood volume of the body is circulated through the kidneys about 300 times each day.
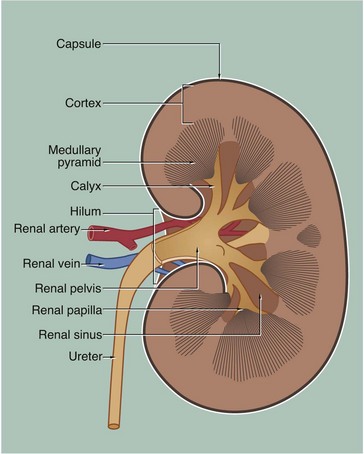
The kidney is a bean-shaped organ lying in the upper retroperitoneal area and oriented with the concave surface directed medially. In adults, the kidney measures 10 to 12 cm. The hilum is the site of entry and exit of the renal blood vessels and the ureter.
The archetypal kidney of lower mammals consists of a single lobe made up of a medullary pyramid (actually cone-shaped), the base of which is enveloped by the cortex containing the renal corpuscles and the proximal and distal parts of the tubules. Nephrons arise in the cortex, loop down into the medulla and return to the cortex. From here they drain into collecting ducts that descend again into the medulla to discharge urine from the apex of the medullary pyramid. The apical part of the pyramid (known as the renal papilla) is enveloped by a funnel-shaped renal pelvis, which represents the dilated proximal part of the ureter.
The human kidney is made up of 10 to 18 lobes. In the adult, the cortical components of the lobes are fused so that the cortex forms a continuous smooth outer zone which extends down between the pyramids. The renal medulla is made up of multiple medullary pyramids, separated by medullary extensions of the cortex. Each renal papilla is surrounded by a branch of the renal pelvis called a calyx; the whole urinary collecting system within the kidney being described as the pelvicalyceal system. The space between the branches of the pelvicalyceal system is filled with fatty supporting tissue and is known as the renal sinus.
The kidney is invested by a tough fibrous capsule which is surrounded by a thick layer of perinephric fat that is in turn encased in a delicate condensation of connective tissue known as Gerota’s fascia. The fat around the kidney cushions it against trauma.
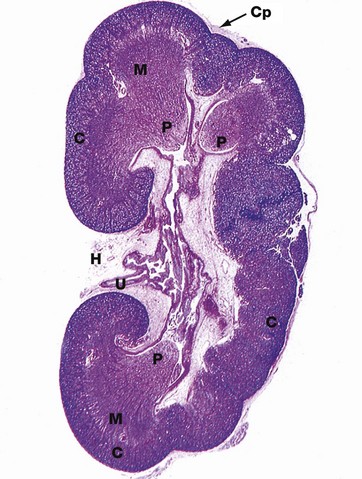
H&E (LP)
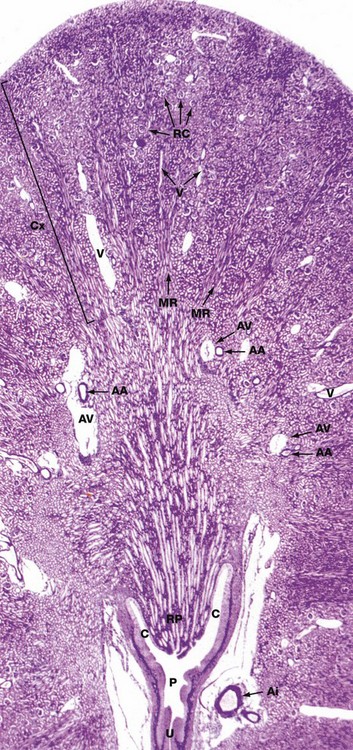
This micrograph of a kidney from a stillborn child illustrates at low power the features of the kidney described in Fig. 16.2. The kidney of a baby has been chosen as it is small enough to section and photograph in its entirety. Furthermore its convex surface is irregular, reflecting the development of the many lobes making up the organ. In histological section, only a single plane through the pelvicalyceal system can be visualised. This plane of section includes the axes of three lobes, the papilla P of each one projecting into the central pelvicalyceal space; this drains into the ureter U that leaves the kidney via the hilum H.
The darker-stained cortex C can be clearly differentiated from the paler-stained medulla M. The cortex contains large numbers of tiny spheroidal structures, the developing renal corpuscles (see Fig. 16.8). The medullary pyramids are characterised by the numerous tubules converging towards the tips of the renal papillae. Note the continuity of the cortex throughout the outer zone of the kidney and the cortical extension between the two medullary pyramids at the top of the field. The fibrous capsule Cp of the kidney is continuous at the hilum with fatty supporting tissue, which packs the space (known as the renal sinus) between the hilar structures. The renal artery and vein also pass through the hilum but are not seen in this plane of section.
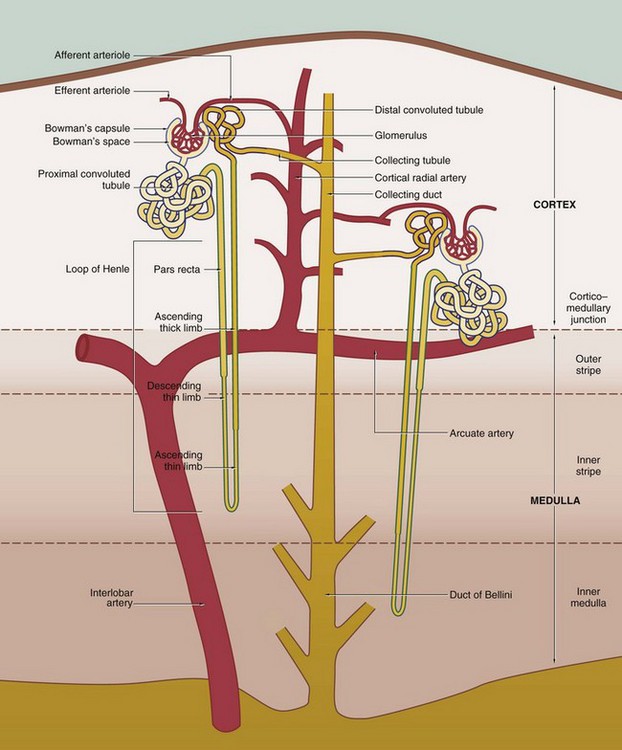
The nephron, the functional unit of the kidney, consists of two major components, the renal corpuscle and the renal tubule. A normal adult kidney contains between 0.6 and 1.2 × 106 nephrons.
Renal corpuscle
The renal corpuscle is responsible for the filtration of plasma and is a combination of two structures, Bowman’s capsule and the glomerulus.
Bowman’s capsule consists of a single layer of flattened cells resting on a basement membrane; it is derived from the distended blind end of the renal tubule. The glomerulus is a globular network of anastomosing capillaries which invaginates Bowman’s capsule (see Fig. 16.7). Thus the capillary loops of the glomerulus are invested by the visceral layer of Bowman’s capsule, a highly specialised layer of epithelial cells called podocytes (see Fig. 16.12). The visceral layer is reflected around the vascular stalk of the glomerulus to become continuous with the parietal layer that constitutes Bowman’s capsule proper. The space between the two layers is known as Bowman’s space and is continuous with the lumen of the renal tubule; the parietal epithelium of Bowman’s capsule is continuous with the epithelium lining the renal tubule.
In the renal corpuscle, water and low molecular weight constituents of plasma are filtered from the glomerular capillaries into Bowman’s space to form the glomerular ultrafiltrate, which then passes into the renal tubule. Thus the filtration barrier between the capillary lumen and Bowman’s space consists of the capillary endothelium, the podocyte layer and their common basement membrane known as the glomerular basement membrane (see Fig. 16.14); these three components are sometimes called the glomerular filtration barrier.
The afferent arteriole, which supplies the glomerulus, and the efferent arteriole, which drains it, enter and leave the corpuscle at the vascular pole that is usually situated opposite the entrance to the renal tubule, the urinary pole (see Fig. 16.8).
Renal tubule
The renal tubule extends from Bowman’s capsule to its junction with a collecting duct. The renal tubule is up to 55 mm long in humans and is lined by a single layer of epithelial cells. The primary function of the renal tubule is the selective reabsorption of water, inorganic ions and other molecules from the glomerular filtrate. In addition, some inorganic ions are secreted directly from blood into the lumen of the tubule. In humans, glomerular filtrate is produced at a steady rate of approximately 120 mL/min; of this, all but about 1 mL is reabsorbed by the renal tubules, giving a normal rate of urine production of around 1mL/min. The renal tubule has a convoluted shape and has four distinct zones, each of which has a different role in tubular function and a corresponding difference in histological appearance.
In most cases, each kidney is supplied by a single renal artery which divides in the hilum into two main branches; in some individuals, however, there are two or even more renal arteries that derive directly from the aorta. Each of these gives rise to several interlobar arteries which ascend between the pyramids to the corticomedullary junction. Here they branch to form the arcuate arteries, which run in an arc-like course parallel to the capsule of the kidney. The arcuate arteries give rise to numerous interlobular (cortical radial) arteries that radiate towards the capsule, branching to form the afferent arterioles of the glomeruli.
As previously described, the vasa recta form a continuation of the efferent arterioles of juxtamedullary glomeruli and form the microcirculation of the renal medulla. The efferent arterioles of the rest of the cortex divide to form the plexus of capillaries that surround the tubules of the renal cortex. The cortical and medullary capillaries drain via cortical radial (interlobular) veins to arcuate veins at the cortico-medullary junction and thence to the renal vein.
Jones methenamine silver H&E (LP)
The basic geography of the kidney can be seen in this unilobar kidney that has been sectioned through the axis of the medullary pyramid. Note the cup-shaped calyx C surrounding the renal papilla RP. The calyces fuse to form the pelvis P that in turn leads to the ureter U.
In the cortex Cx, numerous renal corpuscles RC (200 µm in diameter) are just visible at this magnification. The corpuscles tend to be arranged in parallel rows at right angles to the capsule, separated by interlobular arteries from which they derive their blood supply. Interlobular arteries are too narrow to be identified at this magnification, but a number of their accompanying thin-walled interlobular veins V are easily seen.
Most of the cortical parenchyma surrounding the renal corpuscles consists of proximal and distal convoluted tubules. From the cortex, medullary rays MR course towards the medulla; they consist of collecting tubules and ducts draining nephrons located high in the cortex. The collecting ducts merge in the medulla to form the larger ducts of Bellini that converge towards the tip of the renal papilla. Although not visible at this magnification, long loops of Henle dip into the medulla between and parallel with the collecting ducts. The long, straight vasa recta also dip down into the medulla alongside the loops of Henle; these vessels, too small to be seen at this magnification, absorb water from the loops of Henle and collecting ducts.
The corticomedullary junction is marked by several arcuate arteries AA and their associated thin-walled arcuate veins AV. Note a large interlobar branch of the renal artery Ai in the hilar supporting tissue.
The Renal Cortex
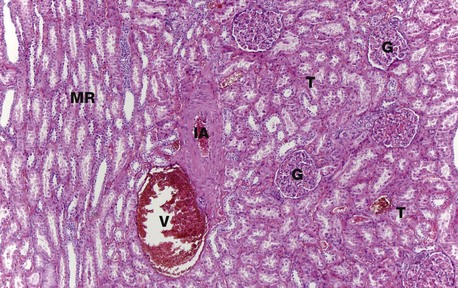
H&E (MP)
At higher magnification, the renal corpuscles are dense rounded structures, the glomeruli G, surrounded by narrow Bowman’s spaces, normally filled with plasma ultrafiltrate and only just visible at this magnification The tubules T fill the bulk of the parenchyma between the corpuscles. The cortex consists mainly of proximal convoluted tubules lined by more eosinophilic epithelial cells, with smaller numbers of distal convoluted tubules and collecting tubules. At the left side of the micrograph, part of a medullary ray MR composed of collecting tubules is easily identified. An interlobular artery IA and vein V are also easily identified.
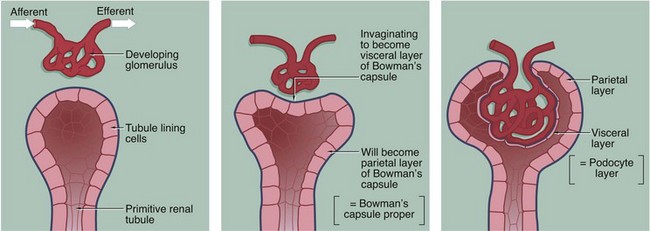
The three-dimensional structure of the renal corpuscle can be clarified by studying its development. The renal tubules develop from the embryological metanephros as blind-ended tubes consisting of a single layer of cuboidal epithelium. The ends of the tubules dilate and become invaginated by a tiny mass of mesoderm tissue that differentiates to form the glomerulus. The layer of invaginated epithelium flattens and differentiates into podocytes that become closely applied to the outer surfaces of glomerular capillaries (the visceral layer of Bowman’s capsule). Most of the intervening tissue disappears so that the basement membranes of glomerular endothelial cells and podocytes effectively fuse, forming the glomerular basement membrane. A small amount of tissue remains to support the capillary loops and differentiates to form the mesangium. Where the mesangium stretches between the capillary loops, its urinary surface is invested by podocyte cytoplasm with underlying basement membrane.
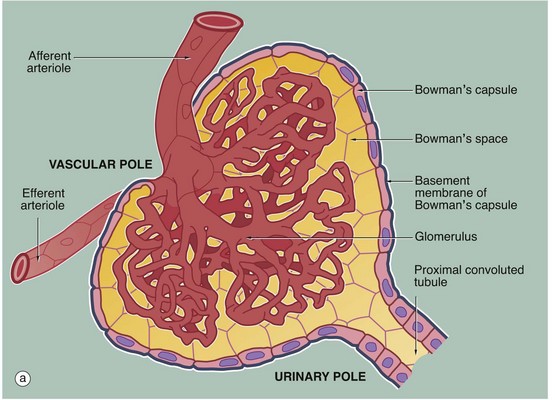
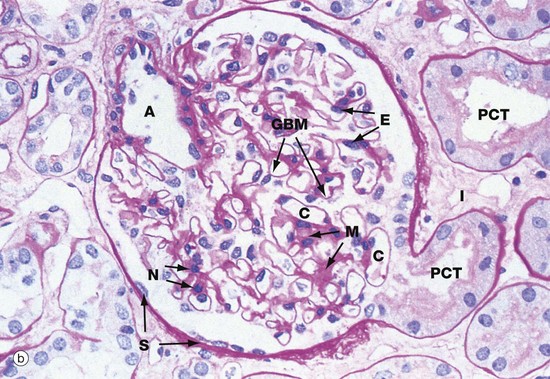
(a) Schematic diagram (b) PAS (HP)
The main structural features of the renal corpuscle are demonstrated in diagram (a). The relatively wide-diameter afferent arteriole enters Bowman’s capsule at the vascular pole of the renal corpuscle and then branches to form an anastomosing network of glomerular capillaries, each major branch giving rise to a lobule. The glomerulus is thus suspended in Bowman’s space from the vascular pole. The spaces between the capillary loops in each glomerular lobule are filled by mesangium which contains mesangial cells (not shown).
The efferent vessel draining the glomerulus is unusual in that it has the structure of an arteriole and is thus called the efferent arteriole (not venule). The efferent arteriole is of smaller diameter than the afferent arteriole, and a pressure gradient is thus maintained that drives the filtration of plasma into Bowman’s space.
The layer of podocytes investing the glomerular capillaries (visceral epithelial cells) is not shown in this diagram. At the vascular pole, the podocyte layer is reflected to become continuous with the parietal epithelial cells of Bowman’s capsule, which in turn becomes continuous with the first part of the renal tubule, the proximal convoluted tubule.
The renal corpuscle in micrograph (b) has been sectioned through the vascular pole and shows the afferent arteriole A entering the glomerulus. The efferent arteriole is not seen in this plane of section. At the urinary pole, the start of the proximal convoluted tubule PCT is seen. Other proximal convoluted tubules can be seen cut in various planes of section embedded in the renal interstitium I. Glomerular capillaries C are cut in transverse, longitudinal and oblique sections. The numerous nuclei in the glomerulus are those of capillary endothelial cells, mesangial cells and podocytes.
The PAS stain picks out the glomerular basement membrane GBM and the mesangium M, which consists of basement membrane–like material. Mesangial cells are found embedded within the mesangium, but only their nuclei N can be discerned at this magnification. The capillary lumina are lined by endothelial cells, again only identifiable by their nuclei E.
Note the flattened nuclei of the parietal epithelial cells S lining Bowman’s capsule. This squamous epithelium is continuous with the epithelium of the proximal convoluted tubule and undergoes an abrupt transition to cuboidal form at the urinary pole. The basement membrane of Bowman’s capsule is a thick basement membrane which is highlighted by the PAS stain; it is most likely synthesised by the overlying epithelial cells. Bowman’s capsule is a permeability barrier preventing escape of the plasma ultrafiltrate into the interstitium. The epithelial cells are connected by tight junctions and also contribute to the permeability barrier.
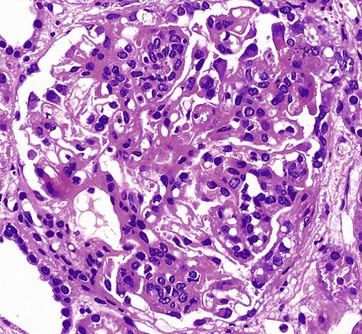
H&E (HP)
This glomerulus is from a patient with glomerulonephritis. Compare this glomerulus with the normal one in Fig. 16.8. Note how the glomerulus seems full of cells and is virtually solid. The glomerular capillary loops are partially obstructed by a mixture of activated endothelial cells and mesangial cells. Less obvious with this staining method is the thickening of the glomerular basement membrane. This patient has the autoimmune disease systemic lupus erythematosus (SLE), and the histological changes in the glomerulus result from the deposition of immune complexes and the response of the intrinsic glomerular cells to these immune complexes. The immune complexes consist of antibodies and antigen, such as double-stranded DNA, a normal body component. The clinical symptoms and signs of lupus nephritis include haematuria, proteinuria including nephrotic syndrome, hypertension and, in some cases, eventual chronic renal failure.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine
