14 URINARY SYSTEM
The urinary system has three critical functions: (1) to clear the blood of nitrogenous and other waste metabolic products by filtration and excretion; (2) to balance the concentration of body fluids and electrolytes, also by filtration and excretion; and (3) to recover by reabsorption small molecules (amino acids, glucose, and peptides), ions (Na+, C1−, Ca2+, PO3–), and water, in order to maintain blood homeostasis (Greek homoios, similar; stasis, standing).
The kidneys is also an endocrine organ. It produces erythropoietin, a stimulant of red blood cell production in bone marrow (for the role of erythropoietin, see Chapter 6, Blood and Hematopoiesis). It also activates 1,25-hydroxycholecalciferol, a vitamin D derivative involved in the control of calcium metabolism (see vitamin D metabolism in Chapter 19, Endocrine System).
Organization of the renal vascular system
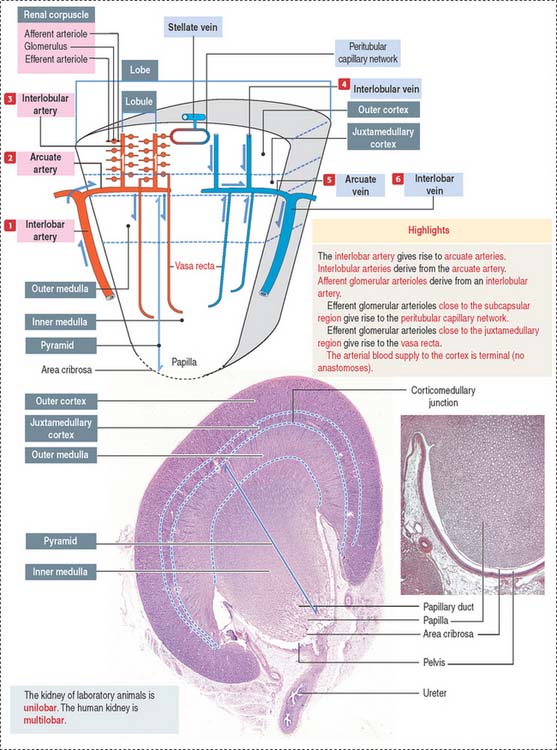
We start our discussion by focusing on the vascularization of the kidneys (Figure 14-1).
At the corticomedullary junction, interlobar arteries give off several branches at right angles, changing their vertical path to a horizontal direction to form the arcuate arteries, running along the corticomedullary boundary. The renal arterial architecture is terminal. There are no anastomoses between interlobular arteries. This is an important concept in renal pathology for understanding focal necrosis as a consequence of an arterial obstruction. For example, renal infarct can be caused by atherosclerotic plaques in the renal artery or embolization of atherosclerotic plaques in the aorta.
Vertical branches emerging from the arcuate arteries, the interlobular arteries, penetrate the cortex. As interlobular arteries ascend toward the outer cortex, they branch several times to form the afferent glomerular arterioles (see Figure 14-1).
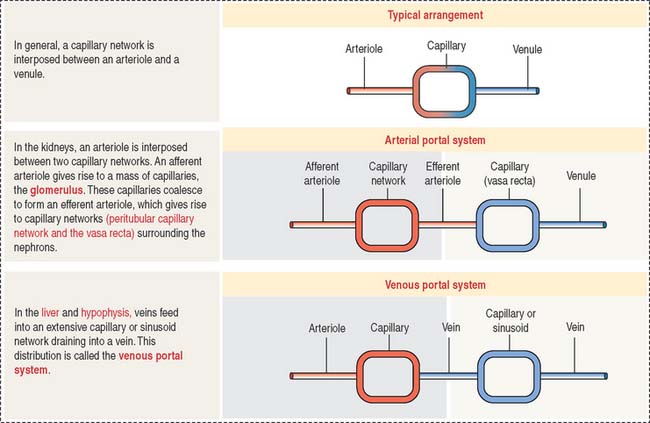
The afferent glomerular arteriole, in turn, forms the glomerular capillary network, enveloped by the two-layered capsule of Bowman, and continues as the efferent glomerular arteriole. This particular arrangement, a capillary network flanked by two arterioles (instead of an arteriole and a venule) is called the glomerulus or arterial portal system. As discussed in Chapter 12, Cardiovascular System, the glomerular arterial portal system (Figure 14-2) is structurally and functionally distinct from the venous portal system of the liver.
Vasa recta
Difference between lobe and lobule
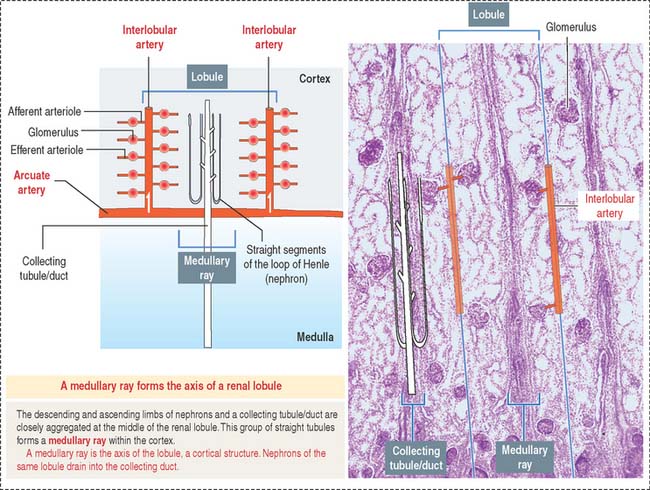
A renal lobule is a cortical structure that can be defined in two different ways (see Figure 14-1): (1) The renal lobule is a portion of the cortex flanked by two adjacent ascending interlobular arteries. Each interlobular artery gives rise to a series of glomeruli, each consisting of an afferent glomerular arteriole, a capillary network, and the efferent glomerular arteriole. (2) The renal lobule consists of a single collecting duct (of Bellini) and the surrounding nephrons that drain into it. The straight portions of the nephrons, together with the single collecting duct, is called a medullary ray (of Ferrein). A medullary ray is the axis of the lobule (Figure 14-3).
Note that the cortex has many lobules and that each lobule has a single medullary ray.
The uriniferous tubule consists of a nephron and a collecting duct
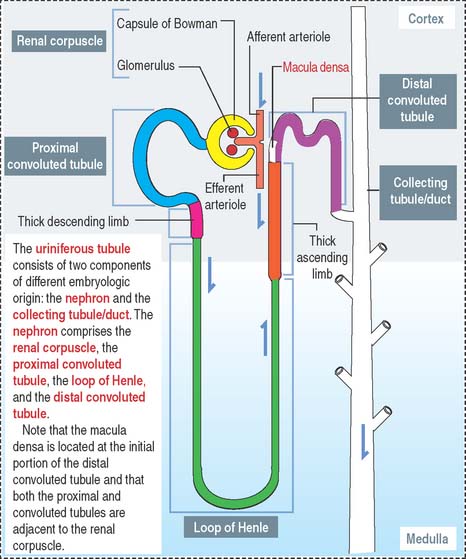
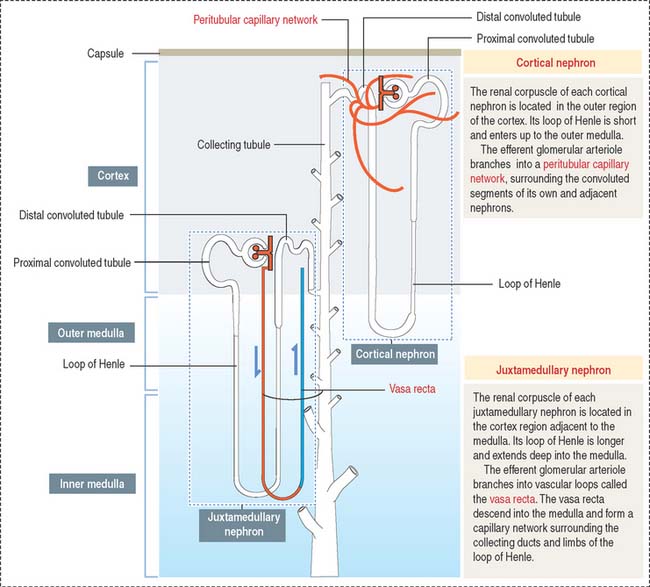
Each kidneys has about 1.3 million uriniferous tubules surrounded by a stroma containing loose connective tissue, blood vessels, lymphatics, and nerves. Each uriniferous tubule consists of two embryologically distinct segments (Figure 14-4): (1) the nephron and (2) the collecting duct.
Depending on the distribution of renal corpuscles, nephrons can be either cortical or juxtamedullary. Renal tubules derived from cortical nephrons have a short loop of Henle that penetrates just up to the outer medulla. Renal tubules from juxtamedullary nephrons have a long loop of Henle projecting into the inner medulla (Figure 14-5).
The renal corpuscle
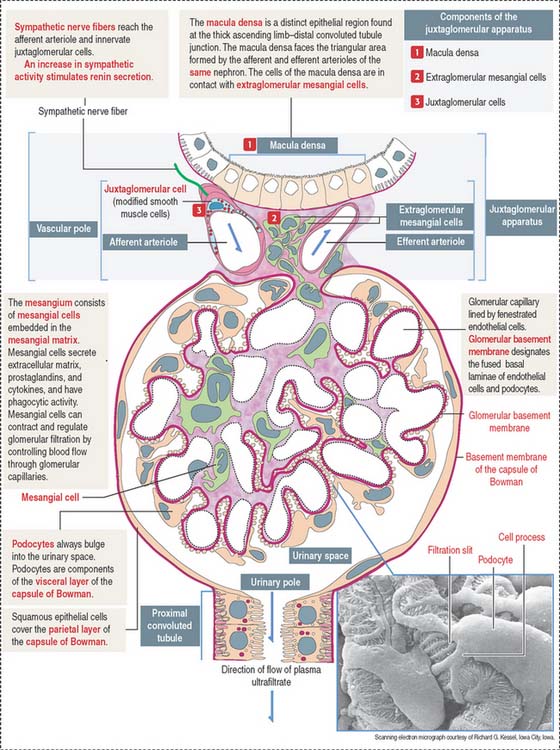
The renal corpuscle, or malpighian corpuscle (Figure 14-6), consists of the capsule of Bowman investing a capillary tuft, the glomerulus.
A urinary space (Bowman’s space or capsular space), containing the plasma ultrafiltrate (primary urine), exists between the visceral and parietal layers of the capsule. The plasma ultrafiltrate contains trace amounts of protein. The urinary space is continuous with the lumen of the proximal convoluted tubule at the urinary pole, the gate through which the plasma ultrafiltrate flows into the proximal convoluted tubule. The opposite pole, the site of entry and exit of the afferent and efferent glomerular arterioles, is called the vascular pole.
The glomerulus consists of two components (Figure 14-7):
The renal corpuscle: Glomerular filtration barrier
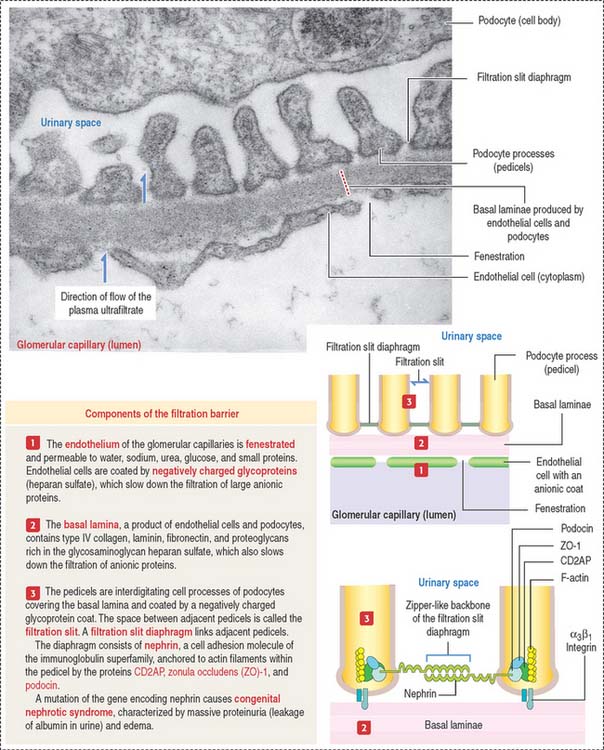
The endings of the cell processes, the pedicels, from the same podocyte or adjacent podocytes, interdigitate to cover the basal lamina and are separated by gaps, the filtration slits. Filtration slits are bridged by a membranous material, the filtration slit diaphragm (Figure 14-8). Pedicels are attached to the basal lamina by α3β1 integrin.
Clinical significance: Glomerular filtration defects
The fenestrated endothelial cells of the glomerular capillaries are covered by a basal lamina to which the foot processes of the podocytes attach (see Figure 14-8). Podocytes produce glomerular endothelial growth factor to stimulate the development of the endothelium and maintenance of its fenestrations.
Type IV collagens are directly involved in the pathogenesis of three diseases. (1) Goodpasture syndrome, an autoimmune disorder consisting in progressive glomerulonephritis and pulmonary hemorrhage, caused by anti-α3(IV) antibodies binding to the glomerular and alveolar basal laminae. (2) Alport’s syndrome, a progressive inherited nephropathy, characterized by irregular thinning, thickening, and splitting of the glomerular basal lamina. Alport’s syndrome is transmitted by an X-linked recessive trait, is predominant in males, and involves mutations of the COL4A5 gene. Patients with Alport’s syndrome—often associated with hearing loss (defective function of the stria vascularis of the cochlea) and ocular symptoms (defect of the lens capsule)—have hematuria (blood in the urine) and progressive glomerulonephritis leading to renal failure. The abnormal glomerular filtration membrane enables the passage of red blood cells and proteins. (3) Benign familial hematuria, caused by a dominant inherited mutation of the COL4A4 gene, which does not lead to renal failure.
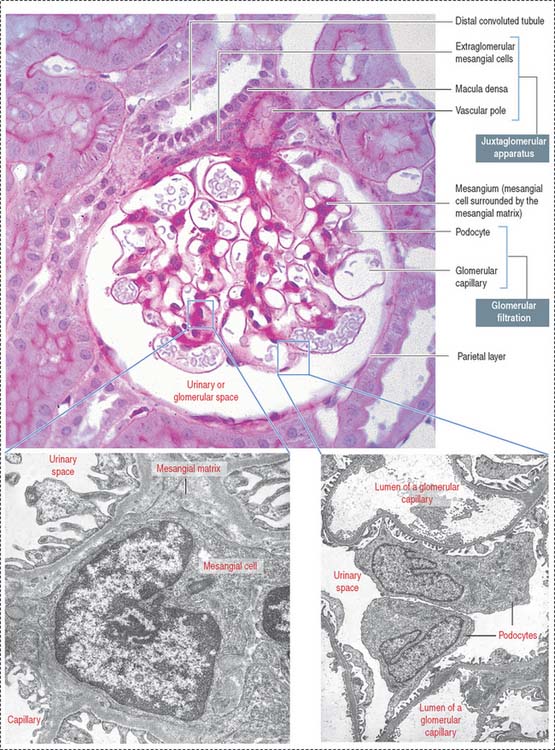
Mesangium
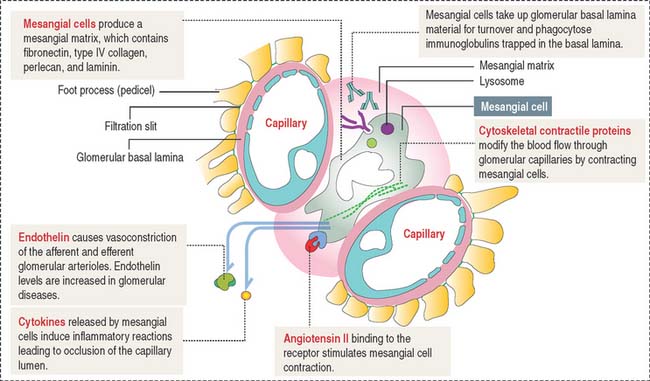
The mesangium, an intraglomerular structure interposed between the glomerular capillaries, consists of two components: (1) the mesangial cells and (2) the mesangial matrix. In addition, mesangial cells aggregate outside the glomerulus (extra-glomerular mesangial cells; see Figures 14-7 and 14-15) in a space limited by the macula densa and the afferent and efferent glomerular arterioles. Intraglomerular mesangial cells may be continuous with extraglomerular mesangial cells.
Mesangial cells participate indirectly in the glomerular filtration process by:
The glomerular filtration membrane does not completely surround the capillaries (Figure 14-9). Immunoglobulins and complement molecules, unable to cross the filtration barrier, can enter the mesangial matrix. The accumulation of immunoglobulin complexes in the matrix induces the production of cytokines by mesangial cells that trigger an immune response leading to the eventual occlusion of the glomerulus.
Clinical significance: Immuno mediated glomerular diseases
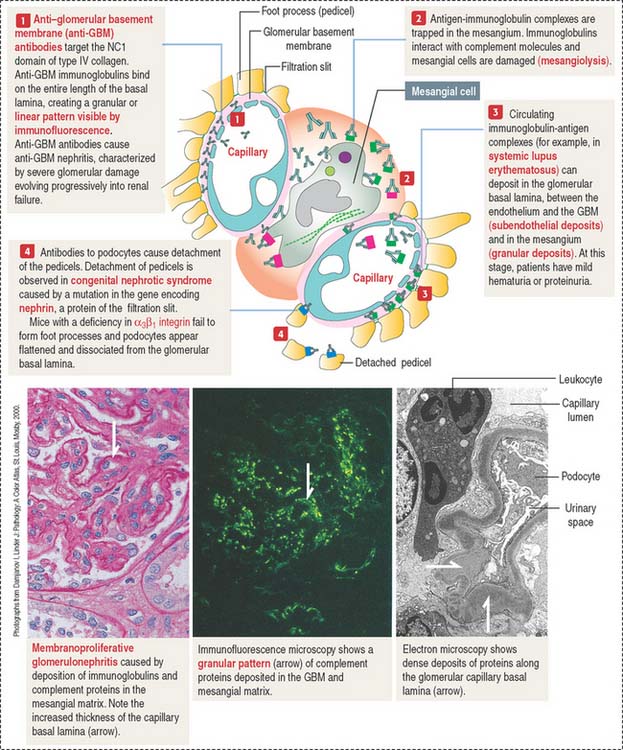
The damage to the glomerulus can be initiated by immune mechanisms. Antibodies against glomerular components (cells and basal lamina) and antibody-complement complexes circulating in blood in patients with systemic autoimmune diseases can cause glomerular injury such as membranoproliferative glomerulonephritis (Figure 14-10), membranous glomerulonephritis and immunoglobulin A nephropathy (Berger’s disease).
Antibody-antigen complexes are not immunologically targeted to glomerular components. They are trapped in the glomerulus because of the filtration properties of the glomerular filtration barrier. A complicating factor is that trapped antibody-antigen complexes provide binding sites to complement proteins, which also contribute to the glomerular damage (see Chapter 10, Immune-Lymphatic System, for a review of the complement cascade).
Immune complexes can deposit between the endothelial cells of the glomerular capillaries and the basal lamina (subendothelial deposits, see Figure 14-10), in the mesangium, and less frequently between the basal lamina and the foot processes of podocytes (subepithelial deposits).
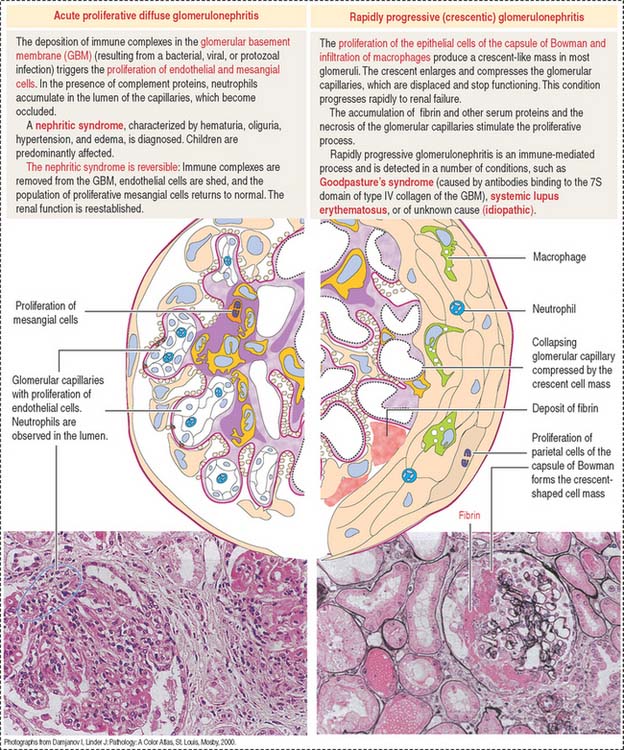
Immune complexes produced after bacterial infection can cause the proliferation of glomerular cells (endothelial and mesangial cells) and attract neutrophils and monocytes. This condition, known as acute proliferative glomerulonephritis, is observed in children and is generally reversible with treatment. This disease is more severe in adults: it can evolve into rapidly progressive (crescentic) glomerulonephritis (Figure 14-11).