Purine metabolism and uric acid
Purines are simple, cyclic organic molecules which are essential constituents of the nucleic acids, both DNA and RNA. The purine bases adenine and guanine comprise the ‘A’ and ‘G’ of the DNA code with the ‘C’ and ‘T’ contributed by the related pyrimidine bases, cytidine and thymine. When a ribose sugar moiety is linked to the purine base a nucleoside is formed (e.g. adenosine, made up of the purine adenine linked to ribose). The addition of a phosphate group to the ribose ring generates the corresponding nucleotide (e.g. adenosine 5-monophosphate, AMP). As such the purines are essential constituents of metabolically important compounds such as ATP.
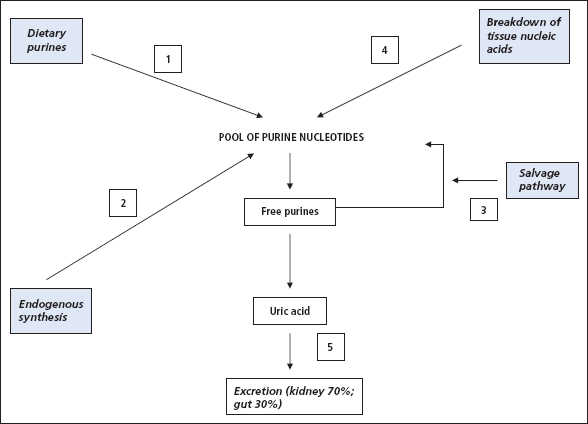
Figure 18.1 shows that uric acid is the end-product of breakdown of the purine bases. It emphasises that the source of the purines can be from the three routes of diet, nucleic acid breakdown or de novo purine synthesis. Once formed, uric acid is predominantly excreted via the kidneys (~70%) with some excretion via the intestine. At physiological pH, uric acid is almost completely ionised, circulating as the urate anion.
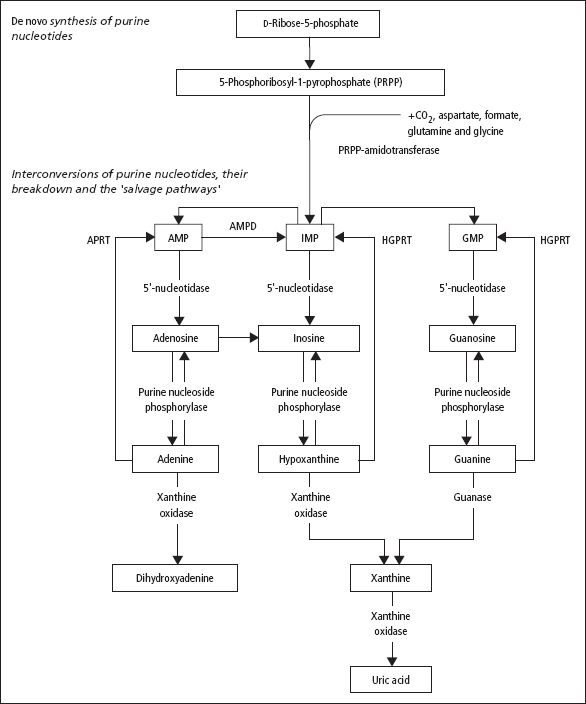
Fig. 18.2 summarises the de novo synthetic route for purines from the simpe activated ribose molecule, 5-phosphoribosyl-1-pyrophosphate (PRPP). The purine ring is built onto this sugar, and the nucleotide which is first formed (IMP; see Figure 18.2) can be converted to both AMP and GMP nucleotides. It also illustrates that the pathway to uric acid can be reversed at some points (converting the free purine bases to corresponding nucleotides), the so-called salvage pathway. The nucleotides produced by the salvage pathway can be re-incorporated into nucleic acids.
Serum urate
Serum [urate] may be increased (hyperuricaemia) as a result of either excessive formation, reduced excretion or a combination of both. (Figure 18.1). Other factors can also influence these underlying routes of serum [urate] accummulation. These are:
- Sex Serum levels are higher in males (0.12–0.42 mmol/L) than in females (0.12–0.36 mmol/L).
- Obesity Levels tend to be higher in the obese.
- Social class The more affluent social classes tend to have a higher serum [urate].
- Diet Serum [urate] rises in individuals taking a high-protein diet (especially meat or seafood). High alcohol consumption and fructose-containing beverages are also associated with raised serum [urate]. Dairy foods and coffee drinking appear to lower urate when examined in epidemiological studies.
- Genetic factors.
Figure 18.1 Routes to the formation of uric acid production and examples: (1) purine-rich diet (meats, seafood); (2) 5-phosphoribosyl-1-pyrophosphate synthase overactivity; (3) hypoxanthine-guanine phosphoribosyltransferase deficiency; (4) tumour lysis syndrome; (5) renal failure.
Hyperuricaemia (Table 18.1)
The importance of hyperuricaemia arises because of its potential to cause the condition known as gout, where joint pain and damage arise from the deposition of uric acid crystals in the joints and from the fact that uric acid crystals may be deposited in the renal tract causing renal calculi.
Solutions of monosodium urate become supersaturated when the concentration exceeds 0.42 mmol/L. However, the relationship between the presence and severity of hyperuricaemia and the development of arthritis or renal calculi is more complex than simple considerations of solubility might suggest.
Dietary factors
High purine diets. A high meat diet or one rich in seafood increases the purine load. The protein content per se does not appear to be responsible. For example, dairy foods have a relatively high protein content with low purine content and may actually lower serum [urate], possibly as a consequence of a uricosuric effect of protein.
- Alcohol excess. The image of the gout-ridden individual consuming high levels of alcohol is well known, and nutritional surveys have established a strong link between hyperuricaemia and alcohol intake. The mechanism is probably multifactorial, including an increased nucleotide breakdown, diuresis, dehydration and the influence of lactic acids and ketone bodies (arising from the effects of alcohol metabolism) in reducing [urate] excretion.
- Fructose-containing beverages. A possible mechanism is the consumption of ATP through the fructokinase reaction, with the ADP formed reconverted to ATP in the adenylate kinase reaction, generating AMP which serves as a precursor for uric acid (Fig. 18.2).
Figure 18.2 The upper part of this figure is a simplified representation of the synthetic pathway leading to the de novo synthesis of inosinic acid (IMP), a purine nucleotide that can then be converted to adenylic acid (AMP) and guanylic acid (GMP) by complex reactions, indicated by the upper pair of curved arrows. The lower part of the figure shows the breakdown of AMP, IMP and GMP to the corresponding purines, and their further metabolism to uric acid (the main end-product of purine metabolism) and dihydroxyadenine. It also shows, with another set of curved arrows, the salvage pathways for re-forming AMP, IMP and GMP from their corresponding purine bases, by reactions catalysed by hypoxanthine-guanine phosphoribosyltransferase (HGPRT) and by adenosine phosphoribosyl transferse (APRT).
Endogenous overproduction of urate
A number of mechanisms are possible. For example:
- Unspecified overactivity of the pathways of nucleotide metabolism, as opposed to nucleic acid synthesis, leading to urate formation (‘endogenous overproduction’).
- Decreased activity of the ‘salvage’ pathway so that purine bases are metabolised to urate rather than re-incorporated into nucleotides and nucleic acids.
- Increased nucleic acid breakdown when cell turnover or destruction is increased.
Defective elimination of urate
Renal excretion of urate is a complex process. Except for a small fraction bound to plasma proteins, urate is completely filtered at the glomerulus; this is then mostly reabsorbed in the proximal tubule. In the distal tubule, there is both active secretion and post-secretory reabsorption at a more distal site. These processes can all be affected by disease or drugs:
- GFR When the GFR becomes reduced for any reason (p. 56), urate retention occurs.
- Distal tubular secretion Lactic acid, 3-hydroxy-butyric acid and some drugs (e.g. thiazide diuretics) compete with urate for this excretory pathway. Any condition giving rise to lactic acidosis or ketosis tends to be associated with hyperuricaemia.
- Distal tubular reabsorption

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


