Fig. 6.1
Anatomy of upper respiratory tract
Functionally, respiratory system is divided into the conducting and the gas exchanging parts. The nose, mouth, pharynx, larynx, trachea and bronchi are various parts of conducting airways. The respiratory bronchioles and alveoli compose the gas exchanging part of the lungs. Thus, upper respiratory tract is a part of conducting airways in the respiratory system (Gaga et al. 2001).
Nose is the first part of upper airways which is divided into two cavities by nasal septum. These two cavities again at the nasopharynx level join together and form a unique airway. Nasal vestibules are the most anterior parts of the nasal cavity. They are narrowing towards the main nasal cavity in junction with the main nasal cavity making the narrowest part of the airways, which is called nasal valve. Nasal vestibules are enclosed by the cartilages of nose and are covered by stratified squamous epithelium and contain hairs (vibrissae) and sebaceous glands. The small hairs of vestibules act as a filter and remove any large dust particles in the inspirated air. These short stiff hairs are exceedingly sensitive to certain mechanical stimuli and respond immediately to the stimulation with itching and sneeze, protecting and notifying (Gaga et al. 2001).
In the main nasal cavity, there are three bony structures protruding from the lateral wall on each side which are known as the nasal turbinates or conchae. Inferior, middle and superior turbinates increase the surface of the nose whereas at the same time narrow the lumen. These structures facilitate close contact of inhaled air with the nasal mucosa, and promote humidification and warming of the air. Also, nasal turbinates by air conditioning and shaping the nasal airway provide air flow turbulence and increase deposition and trapping of the particles on to the nasal mucosa. Therefore, the air that is delivered to the lower airways is filtered and conditioned (Mygind et al. 1990).
The olfactory region has been placed in the upper part of the nasal cavity. Paranasal sinuses are air-filled spaces located within the bones of the skull and face around the nasal cavity (Fig. 6.2). They communicate with nasal cavity and provide voice resonance and possibly heat and cold insulation (Blanton and Biggs 1969).
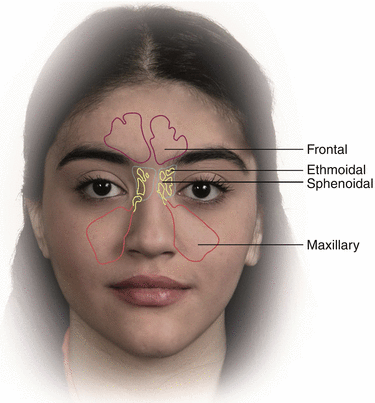

Fig. 6.2
Anatomy of nasal sinuses
The surface of the paranasal sinuses is covered with ciliated pseudostratified columnar epithelium.
Formation of paranasal sinuses begins in the fetus by excavation of bone and air-filled spaces from the nasal cavity. This process continues and completes after birth during the course of growth and maturity. Four paranasal sinuses in human are maxillary, sphenoid, ethmoid and frontal sinuses that maxillary sinuses are the largest sinuses (Rhys Evans 1987).
The next part of the upper airways after the nose is the pharynx. This structure consists of the nasopharynx, oropharynx, and hypopharynx. The nasopharynx begins from the choanae down to the lower margin of the soft palate. The oropharynx, which is located behind the oral cavity extends from the soft palate to the tip of the epiglottis inferiorly. The hypopharynx extends from the upper margin of the epiglottis to the lower border of the cricoids cartilage, serving as the channel from the oropharynx to the laryngeal inlet and esophagus (Kimoff 2005).
The openings of the Eustachian tubes, the adenoids and the tonsils are located in the pharynx.
The pharynx is involved in both the digestive and respiratory tracts and directs the food to the oesophagus and to the stomach and the air to the trachea and lungs.
The last part of the upper respiratory tract after the pharynx is the larynx. The larynx after the nasal valve is the second narrowest part of the airway. It is the organ of phonation and acts as a valve that protects the lower airways and the lungs (Fig. 6.3). The vocal cords and several cartilages are located in the larynx. The largest cartilage found in the larynx is the thyroid cartilage, which produces “Adam’s Apple” prominence on the front of the neck. Another cartilage is the epiglottis that lies on top of the larynx and prevents entrance of the food to the trachea during swallowing. The laryngeal mucosa is loosely bound to the supporting cartilage (Gaga et al. 2001).
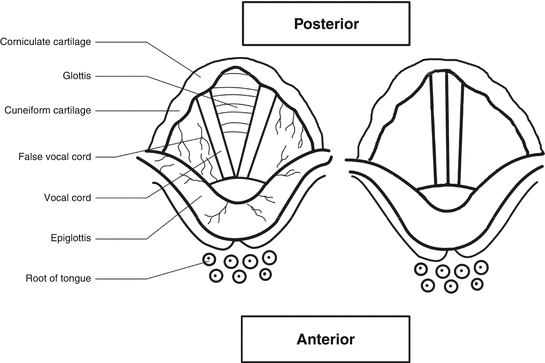

Fig. 6.3
Anatomy of Larynx
Below the larynx, lower respiratory tract begins with trachea which is supported by irregular rings of cartilage that are incomplete dorsally. These cartilages prevent the trachea from collapsing during the rise of intrathoracic pressure. The trachea at its distal end bifurcates to the two main bronchi. The main bronchi also are divided and keep branching and make smaller airways. From the trachea to the alveoli 8–23 generations of airways may exist. The cartilaginous rings yet are present in the main bronchi but they are scarce in the small and more distal airways while no cartilage is found in the bronchioles. The conducting airways end at the terminal bronchioles. After these, respiratory bronchioles and alveoli are present that constitute the gas exchanging unit of the lung (Gaga et al. 2001).
6.3 Clinical Features
Since SM is very lipophilic, it can easily penetrate epithelial tissues and cause marked local damage as well as severe systemic intoxication (Kehe and Szinicz 2005). SM has bidirectional effects; a direct effect via inhalation, and an indirect effect by recirculation. Studies using whole-body autographic with S35-labeled SM have shown increased radioactivity in the nasal region after percutaneous or intravenous administration (Clemedson et al. 1963).
The eyes, nasal mucosa, throat, pulmonary tract, and skin are the most commonly affected sites of body by SM. These organs are the main targets for direct toxic effects of SM (Ghanei et al. 2006b).
As SM is dispersed in the form of aerosol or vapor (Borak and Sidell 1992), it enters the body by inhalation and the first contact area of inhaled toxins with respiratory tract is the nasal and oral mucosa. Most of the SM is absorbed in the upper airways and little reaches the lung parenchyma and alveoli. This mechanism protects the lung tissue against toxic effects of SM but causes upper airway diseases. SM deeply affects respiratory tract from its initial contact area of nasal and oral cavity to the vulnerable surfaces of distal respiratory targets of the pulmonary tree.
The special nature of the respiratory system mucosal membranes, the rapid turnover of its epithelium, the large surface area of the respiratory tract and the oily nature and persistency of the SM (Vander et al. 1998), all cause susceptibility of the respiratory tract to the toxic effects of SM (Graham and Schoneboom 2013).
Epithelial cells of respiratory tract are extremely vulnerable to the toxic effects of mustard gas.
The main injures of respiratory damage by SM are sloughing of the epithelial cells and increases in production of the secretions in the entire respiratory tract. These changes cause nasal discharge, bronchiolar obstruction, and even bronchospasm. These events may interfere with gas exchange at the alveolar level, which can result in hypoxia, hypercarbia, and respiratory and metabolic acidosis (Borak and Sidell 1992; Kehe and Szinicz 2005; Haber 1986).
Effects of SM in the respiratory tract from the nasal mucosa to the terminal bronchioles are dose dependent (Hefazi et al. 2005; Balali-Mood and Hefazi 2006) and the inhalation dose depends on the respiration rate; the higher respiratory rate, the higher inhalation doses (Maynard 2007). Severity of intoxication can be different based on the age, duration and frequency of exposure, gas concentration and quantity, environmental temperature, and the use of protective equipments.
SM can induce early (acute) and late (chronic) complications in the upper respiratory tract (Rowell et al. 2009; Bijani and Moghadamnia 2002).
6.3.1 Early Clinical Features of the Upper Respiratory Tract After Mustard Gas Exposure
The data about early effects of SM on upper airways are scares and there is only one study available in this regards, is from the Iraq-Iran battle (Kehe et al. 2009).
Potent acids, alkalies, mustard gas, phenols, cresols orphosphorus can cause chemical burns.
Characteristics of burn injury in the upper respiratory tract are different from those in the bronchus and lung parenchyma. Chemical burn complications in the upper airway usually develop late with tracheal stenosis after a symptom-free period, unlike the lower respiratory tract injury, which manifests soon after burns (Yang et al. 1999). Symptoms of burn injury in the upper respiratory tract include aphonia, wet or breathy voice quality and inability to initiate a swallow (Pore and Reed 1997).
Due to the high chemical reactivity of mustard gas, most of the acute damages are limited to the upper respiratory tract (Iwaszkiewicz 1966). In the acute phase of exposure mustard gas has direct contact with upper airway mucosa and irritates them directly. Acute damage to the respiratory tract causes acute edema, inflammation, and destruction of the airway epithelial which its severity is different based on the exposure dosage (Pechura and Rall 1993).
In high exposure doses, the clinical respiratory effects of SM after inhalation include an immediate phase of coughing and choking. Upper and lower airways edema with ulcerations and necrosis and tracheobronchitis may develop also in severe exposure usually several hours after exposure (Kehe and Szinicz 2005).
In exposure to moderate SM doses, rhinorrhea, loss of smell and taste, nose and throat discharge and lacrimation are the main observed symptoms (Kehe and Szinicz 2005).
In lower exposure doses, acute respiratory damage occur but the symptoms do not appear immediately and usually there is a brief symptom free delayed period of few hours which is followed by the development of a variety of acute respiratory symptoms such as rhinorrhea, pain, nose, sinus and pharynx discomfort, sinusitis, sinus pain, sneezing, and sore throat as well as respiratory irritation symptoms including dyspnea, tachypnea, coughing and choking and dysphonia. The early respiratory symptoms usually develop 2–16 h after exposure. Rhinorrhea is common symptom but bleeding from the nasal mucosa is rare (Borak and Sidell 1992; Tang and Loke 2012; Kehe and Szinicz 2005; Miller and Chang 2003). Hoarseness, dry cough and sputum production are other symptoms that could develop following acute inhalation of SM (Iwaszkiewicz 1996; Balali-Mood 1986; Taghadosi et al. 2002).
Early symptoms appear in chronological orders based on the dose and mode of exposure, the environmental temperature, the extent of use of protective masks, and the age. Rhinorrhea, sneezing, and sore throat usually develops within 2–6 h of exposure. Aphonia, hoarseness, and non-productive cough appear after 6–24 h and productive cough develops in 24–48 h after exposure. Respiratory problems, improve slowly, although some cough and weak hoarseness may remain for as long as 6 weeks (Balali-Mood and Hefazi 2006; Papirmeister et al. 1991). However, it may take longer time (1–2 months) to recover, particularly after secondary infections and necrotic bronchopneumonia (Papirmeister et al. 1991).
Based on the inhaled dosage, the damage can be mild to severe. Severe damage induce epithelial destruction and sloughing and subsequent formation of pseudomembranes, which may progress to airway obstruction and result in death (Pechura and Rall 1993). These pathologic changes in severe cases are manifested with pulmonary edema, respiratory failure and death in less than 4 % of the patients (Borak and Sidell 1992; Kehe and Szinicz 2005; Haber 1986). Inhalation of higher concentrations of vapor induce laryngeal damage with aphonia or husky voice and injury to the upper medium-sized airways with tracheobronchitis, which usually occurs several hours after exposure (Ghanei et al. 2006a) and is presented by a nonproductive hacking cough (Mx 2003).
In a study on acute effects of SM in chemical victims of Iraq-Iran war, 12 Iranian victims were evaluated in Germany. These victims arrived in Munich 6–8 days (17 days in one case) after exposure and were treated in 3 hospitals during 1984–1985 (Kehe et al. 2009). The patients’ distance from explosion was 5–30 m and none of them had used protective equipment. In this study there was no relationship between the age and the course of disease. The most common early clinical effects of SM exposure in the upper respiratory tract were hoarseness, sore throat and productive cough that were observed almost in all patients. Less common respiratory symptoms were purulent sputum (8/12) and bloody sputum (5/12). Tracheal stenosis occurred in two patients with 10 and 60 % occlusion of the lumen. Twenty percent of patients required tracheotomy (Kehe et al. 2009).
Pathologic changes detected in upper airways were edema (45 %), inflammation (27 %) and obliterative necrosis (27 %). Also, it was found that the healing process in the bronchial tract lesions is faster than that of the throat (Kehe et al. 2009).
Table 6.1 shows the manifestations of upper respiratory effects of SM reported in different studies.
Table 6.1
Manifestations of Upper respiratory tract complications of Sulfur Mustard exposure in different studies
Upper respiratory tract complications Authors, year | Large airway narrowing (%) | Cough (%) | Expectoration (%) | Dysphonia (%) | PND (%) | Laryngitis (%) | Sinuisitis (%) |
|---|---|---|---|---|---|---|---|
Taghadosi et al. (2002) | a | 82.7 | 64.4 | 4.6 | 28.7 | a | a |
Sohrabpour et al. (1988) | a | 91 | 77 | 83 | a | a | a |
Amini and Oghabian (2013) | a | 66 | 33 | a | a | a | a |
Akhavan et al. (2009) | a | 90 | 74 | a | 82 | a | |
Balali-Mood et al. (2010) | a | 97.7 | 88.4 | 79.1 | 41.9 | 14.8 | 55 |
Ghanei et al. (2006b) | a | a | a | a | a | a | a |
Namazi et al. (2009) | 2.72 | 72.38 | 52.98 | a | a | a | a |
Emad and Rezaian (1997) | 9.64 | a | a | a | a | a | a |
Hefazi et al. (2005) | 15 | a | a | a | a | a | a |
Kehe et al. (2009) | 0 | 96.1 | – | – | – | – | – |
24.2 | 100 | a | a | a | a | a | |
6.3.2 Late Clinical Features of the Upper Respiratory Tract After Mustard Gas Exposure
“Late effects of SM poisoning” refer to all organ dysfunctions and abnormalities that occur several years after the first exposure (Ghanei and Vosoghi 2002; Emad and Rezaian 1997; Easton et al. 1988; Bijani and Moghadamnia 2002).
Unlike early effects, there is big data in the literature about the delayed toxic effects of SM in the respiratory tract which most of them are from the Iraq-Iran war. However, little data is available about SM related late clinical effects in the upper respiratory tract from Iraq-Iran conflict.
Evidences have shown that long-term respiratory effects may occur even in the absence of early-phase symptoms. This suggest that late effects are not necessarily dependent to the presence of acute-phase effects and they develop by independent mechanisms (Pechura and Rall 1993).
Late respiratory complications are the major cause of long-term disability and could occur from a few months to several years after exposure. Most available information on late effects are related to the lungs and lower respiratory airway and there is limited information about long-term effect of SM on upper airways.
Early symptoms in acute phase reduce and subside during a few weeks after acute exposure to SM, but the damages persist and gradually progress into the chronic forms. This condition is progressive and during several years, it will convert to delayed complications.
In the first Iranian report on 236 veterans suffering from SM poisoning, the most common complications were found in the respiratory tract (78 %) followed by the central nervous system (45 %), the skin (41 %) and the eyes (36 %) (Balali-Mood 1986). The patients with mild to severe toxicity were included in the above mentioned study and were evaluated 2–28 months after SM exposure (Balali-Mood 1986).
Khateri et al. (2003) study obtained results somewhat different from Balali-Mood report. In their study on 34,000 Iranians veterans exposed to SM, the most common complications were observed in the lung (42.5 %), eyes (39.5 %) and skin (24.5 %) (Khateri et al. 2003). The difference between these two studies may be due to the difference in the study population as the Balali-Mood patients had severe SM exposure and were evaluated after 2–28 months while most of the patients in Khateri et al. study had mild SM exposure and were evaluated 18–23 years following exposure (Khateri et al. 2003; Balali-Mood 1986). In a study on 43 male veterans by Zojaji et al., the most common affected sites were the lung (95.5 %), peripheral nerves (77 %), the skin (73 %), eyes (68 %), and head and neck (16.2 %), respectively. The results of this study are similar to those of Balali-Mood et al. (Zojaji et al. 2009; Balali-Mood 1986).
Delayed effects of SM in the upper airways are characterized by chronic inflammation of the oral cavity, pharynx and larynx, inflammation and ulceration of the palate, nasopharynx, oropharynx and laryngeal cancer with aphonia (Papirmeister et al. 1991; Akhavan et al. 2009).
Laryngitis is one of the main delayed complications of upper respiratory tract among Iranian chemical veterans (Razavi et al. 2013; WHO 1987). Other delayed complications of respiratory tract include chronic bronchitis, bronchiectasis, asthma, large airway narrowing, and pulmonary fibrosis (Balali-Mood 1986; Emad and Rezaian 1997).
Airway narrowing in the late phase is a sequel of acute damage to the trachea and large airways and occurs due to the scarring or granulation tissue formation in the acute phase. Airway stricture usually develops 2 years after exposure (Balali-Mood et al. 2005; Ghanei et al. 2004a, b).
In the chronic phase, chronic cough and sputum production are the main symptoms of chronic bronchitis in the victims (Emad and Rezaian 1997; Ghanei et al. 2005). The most important causes for chronic cough in the late phase are bronchospasm, postnasal drip syndrome, gastroesophageal reflux disease, bronchiectasis, tracheobronchial collapse and postnasal discharge due to chronic sinusitis (Ghanei et al. 2006b).
In the first study in 1988, late respiratory effects of SM intoxication were investigated in 35 Iranian soldiers 6 weeks to 1 year after SM exposure. The most common upper respiratory tract symptoms were cough in 91 % and dysphonia in 83 % of patients (Sohrabpour et al. 1988).
In a study that evaluated 39 patients with chronic cough exposed to a single high dose of SM, paranasal sinus mucosal abnormalities was identified in 76.9 % of the patients, in which 20.5 % had severe mucosal thickening (Ghanei et al. 2006b).
In another study carried out in Iran (Akhavan et al. 2009), late laryngeal effects of SM was assessed in 50 victims after 20 years of SM exposure. That study found hoarseness in 32 %, intermittent dysphonia in 74 %, and continuous dysphonia in 4 %, harshness in 14 % and chronic laryngitis in 82 % of patients. Unilateral vocal cords paralysis was identified in three patients (6 %) and laryngeal nodules in 12 % of victims. They reported vocal cord paralysis as a long-term neurotoxic effect of SM and synechia and vocal cord nodules as a result of laryngeal and bronchial infections. Also, they concluded that hypertrophy of false vocal cords is probably due to dysfunction of the edematous true vocal cords and dysphonia. This study is the only found report that focused on laryngeal effect of SM poisoning (Akhavan et al. 2009).
Balali-Mood et al. in 2010 assessed delayed toxic effects of SM on respiratory tract in 43 male victims of Iraq-Iran war 20–25 years after poisoning. In their study, dysphonia was found in 79.1 %, post-nasal discharge (PND) in 41.9 %, lower larynx position in 30.2 %, vocal cords limitation in 25.6 % and mucosal inflammation of larynx in 14.8 % of patients and therefore dysphonia and chronic sinusitis were the most common delayed effects of SM in upper respiratory tract (Balali-Mood et al. 2010). Vocal cords paralysis and laryngeal nodules were not detected in their patients. Mucosal inflammation of sinuses was found in 25.9 % of patients in Balali-Mood et al. study while in 79 % in Akhavan et al. report. Balali-Mood and colleagues concluded that most of delayed toxic effects of SM in upper respiratory tracts were inflammatory and infectious complications.
Namazi et al. (2009) studied long-term complications of SM intoxication in 134 chemical veterans about 20 years after exposure in Iraq-Iran battle. In their study, all patients suffered from dyspnea, 72.38 % from coughing, and 52.98 % from expectoration (Namazi et al. 2009).
Table 6.2 demonstrates demographic and clinical feature of respiratory complications in different studies in the world.
Table 6.2
Demographic and clinical features of respiratory complications in several studies throughout the world
Authors, year | Country | Number | Population | Mean age | Acute/chronic complications | Duration between exposure and study (years) | Rate of respiratory complications (%) | Upper respiratory tract complications |
|---|---|---|---|---|---|---|---|---|
Taghadosi et al. (2002) | Iran | 87 | Veterans | 35.58 ± 6.45 | Chronic | 12 ± 1.5 | 90.8 | * |
Sohrabpour et al. (1988) | Iran | 35 | Veterans | 28 ± 10.4 | Chronic | 6 week-1 year | 100 | + |
Amini and Oghabian (2013) | Iran | 62 | Veterans | 53 ± 6.9 | Chronic | 20 ± 2.4 | 100 | * |
Heydari and Ghanei (2011) | Iran | 19 | Veterans + civilian | 41.32 ± 4.63 | Chronic | >22 | * | + |
Akhavan et al. (2009) | Iran | 50 | Veterans | 46.6 ± 6.8 | Chronic | 20 | 100 | + |
Balali-Mood et al. (2010) | Iran | 43 | Veterans | 50.6 ± 8.9 | Chronic | 20–25 | * | + |
Ghanei et al. (2006b) | Iran | 39 | Veterans | 37.9 ± 7.6 | Chronic | * | 100 | + |
Namazi et al. (2009) | Iran | 134 | Veterans | 37.2 ± 9 | Chronic | 17–22 | 100 | * |
Emad and Rezaian (1997) | Iran | 197 | Veterans | 34.39 ± 5.95 | Chronic | 10 | 100 | * |
Hefazi et al. (2005) | Iran | 40 | Veterans | 43.8 ± 9.8 | Chronic | 16–20 | 100 | * |
Khateri et al. (2003) | Iran | 34,000 | Veterans | 17–30 | Chronic | 13–20 | 42.5 | * |
Ghasemi Boroumand et al. (2008) | Iran | 600 | Civilian population | 19–80 | Chronic | 19 | 45.8 | * |
Ghasemi Broumand et al. (2007) | Iran | 479 | Militaries + civilian | 21–60 | Chronic | * | 32.1 | * |
Etezad-Razavi et al. (2006) | Iran | 40 | Veterans | 43.8 ± 9.8 (32–76) | Chronic | 16–20 | 95 | * |
Kehe et al. (2009) | Germany | 12 | Iranian Veterans | 18–46 | Acute | 4–17 days | 100 | + |
Bijani and Moghadamnia (2002) | Iran | 220 | Veterans | <30 and >60 | Chronic | 6–13 | 100 | * |
Ghanei et al. (2004a) | Iran | 33 | Veterans | 43 ± 8 | Chronic | 16 ± 0.7 | 100 | * |
6.3.3 Linkage of the Early and Late Toxic Effects of SM
In the respiratory tract, the early effects of SM usually progress to chronic effects without disruption (Taghaddosinejad et al. 2011). Unlike the chronic effects on the skin and eyes that recover during the time, respiratory complications usually progress and worsen over the years (Shirazi et al. 1988; Balali-Mood and Hefazi 2006).
6.4 Upper Respiratory Tract Cancer
SM is a mutagenic and alkylating agent, which alkylates DNA. Experimental and human studies have shown that SM is mutagenic and carcinogenic and could induce mutation and chromosomal aberrations in animal model (Papirmeister et al. 1984; Heston 1950; Takeshima et al. 1994).
Carcinogenicity of SM in human also, has been approved and the International Agency for Research on Cancer (IARC) has confirmed SM as a human carcinogen and has known it as a risk factor for occupational lung cancer (Ghanei and Vosoghi 2002; Nishimoto et al. 1998; Perchura and Rall 1993).
SM could induce malignant changes in various organs such as the hematopoietic and respiratory systems.
However, most of available evidences about the mustard induced cancers of the respiratory tract are related to the lung cancer and there is limited evidence about the carcinogenic effects of mustard gas in the upper respiratory tract.
Most of primary data about the carcinogenicity of SM in human was about occupational exposure obtained from workers of chemical factories with prolonged low dose exposure to SM while there was no data on the carcinogenicity of single high- or low- dose SM exposure (Easton et al. 1988; Manning et al. 1981; Wada et al. 1968; Dacre and Goldman 1996). Different studies also revealed increased risk of respiratory tract cancers in the workers of chemical factories producing SM (Wada et al. 1968; Manning et al. 1981; Easton et al. 1988).
Wada et al. (1968) study on 485 men showed a significant increase in death due to the respiratory cancer including the lungs, pharyngeal and nasal cancer (33 cases against 0.9 expected) among former workers of the Japanese poison gas factory (Wada et al. 1968). The risk of the upper airway cancer in their study was 37 times more than the normal population (Wada et al. 1968).
High incidence of cancer of the larynx, pharynx and other upper airways as well as a moderately increased rate of mortality due lung cancer in the former workers of a British SM manufacture was also reported (Manning et al. 1981).
In a cohort study by Easton et al. in 1988, the mortality due to cancer in 3530 men and women employed in the manufacture of mustard gas, highly significant excesses of death was observed due to the cancer of gum and mouth, larynx and pharynx compared to the national death rates of these cancers. Mortality due to the lung cancer was even moderately excessive in comparison to the upper respiratory tract cancers (Easton et al. 1988). The increased rate of death due to cancers of the tongue, salivary gland, and nose was not significant. Also, it was found that the risks for pharyngeal and lung cancer were significantly related to the duration of employment (Easton et al. 1988). They also compared the mortality of World War II Navy veterans with low dose SM exposures to that of veterans without exposure and did not find any increase in the risk of cause-specific mortality (Easton et al. 1988). In their study relative risk for cancers of the pharynx, larynx, lung, and other upper respiratory sites were associated with duration of exposure. Also, the risk of respiratory cancers among production workers was not considerably greater than that of workers at other factory parts.
Nishimoto et al. (1988) in their study on 1632 workers of SM factory found a fivefold increase in the risk of cancer in the workers employed in the production as well as in other factory parts with direct contact with SM (Nishimoto et al. 1988). They observed an excess of cancers in the nasal sinuses, pharynx, and larynx as well. This study again confirmed the Easton et al. study (1988) and showed that the risk of cancer was significantly associated with the duration of exposure. These studies concluded that long time SM exposure is a risk factor for occupational cancers of upper respiratory tract and the lungs. They have also proved a causal relationship between occupational exposure to SM and respiratory cancers.
Evidence on the mutagenicity and carcinogenicity of mustard gas in human also obtained from battlefield exposures and accidents as well (Hosseini-Khalili et al. 2009). Hosseini-Khalili et al. (2009) assessed p53 and KRAS (Kirsten rat sarcoma) mutations in 18 SM victims with lung cancer. They found eight point mutations in p53 but no mutation in KRAS. The frequent p53 mutation in these patients was similar to that frequently observed in workers of factory with prolonged exposure to SM (Hosseini-Khalili et al. 2009).
Although the carcinoma of the nasopharynx and bronchogenic carcinoma were reported in Iranian veterans (Balali 1992) but a later study in 1997 on 197 chemical veterans of Iraq-Iran war could not find any more cases of bronchial carcinoma or other lung cancers in the victims after 10 years of exposure to SM (Emad and Rezaian 1997). In agreement with this study, again another study on the chemical veterans exposed to SM during the World War I failed to show any significant increase in the observed deaths due to the cancer (2.5 % vs. 1.9 % in controls) (Norman 1975).
However, few years later, results of British and American studies showed increased incidence of lung cancer from the World War I battlefield SM exposures (Somani et al. 2001). Gilasi et al., investigated the incidence of cancer among 500 Iranian victims after 18 years of exposure and 500 unexposed soldiers. They could only detect three cases of cancer in exposed group (Gilasi et al. 2006). They found no significant relationship between cancer and acute exposure to SM.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


