9 Unwanted effects and adverse drug reactions
Definitions
The term adverse drug reaction (ADR) should be confined to harmful or seriously unpleasant effects occurring at doses intended for therapeutic (including prophylactic or diagnostic) effect and which call for reduction of dose or withdrawal of the drug and/or forecast hazard from future administration; it is effects of this order that are of importance in evaluating drug-induced disease in the community. The term adverse ‘reaction’ is almost synonymous with adverse ‘effect’, except that an ‘effect’ relates to the drug and a ‘reaction’ to the patient. Both terms should be distinguished from an adverse ‘event’, which is an adverse happening that occurs during exposure to a drug without any assumption being made about its cause (see Prescription event monitoring, p. 52).
Toxicity
implies a direct action of the drug, often at high dose, damaging cells, e.g. liver damage from paracetamol overdose, eighth cranial nerve damage from gentamicin. All drugs, for practical purposes, are toxic in overdose2 and overdose can be absolute or relative; in the latter case an ordinary dose may be administered but may be toxic due to an underlying abnormality in the patient, e.g. disease of the kidney. Mutagenicity, carcinogenicity and teratogenicity (see Index) are special cases of toxicity.
Attribution and degrees of certainty
The following elements are useful in attributing the cause of an adverse event to a drug:
1. The time sequence in relation to taking the drug. The majority of reactions develop soon after exposure. Anaphylactic reactions (within minutes or hours) and hypersensitivity reactions (within weeks) may readily suggest an association, but delayed effects such as carcinogenesis or tardive dyskinesia (after years or even decades) present more difficulty.
2. The effects of withdrawing or reintroducing the drug. Most reactions subside when the drug is discontinued, unless an autoimmune reaction is established, when effects persist. Planned re-exposing a patient to a drug is rarely indicated unless treatment with it is essential and there is no reliable alternative.
3. The relationship to what is already known about the drug. This of course invites questions about consistency with the established pharmacology and toxicology of the drug or related substances.
Degrees of conviction for attributing adverse reactions to drugs may be ascribed as3:
• Definite: time sequence from taking the drug is reasonable; event corresponds to what is known of the drug and is not explicable by concurrent disease or drugs; event ceases on stopping the drug; event returns on restarting the drug (rarely advisable).
• Probable: time sequence is reasonable; event corresponds to what is known of the drug; event ceases on stopping the drug; event not reasonably explained by patient’s disease or other drugs.
• Possible: time sequence is reasonable; event corresponds to what is known of the drug; uncertain relationship to effect of stopping the drug; event could readily have been result of the patient’s disease or other therapy.
• Conditional: time sequence is reasonable; event does not correspond to what is known of the drug; event could not reasonably be explained by the patient’s disease or other drugs.
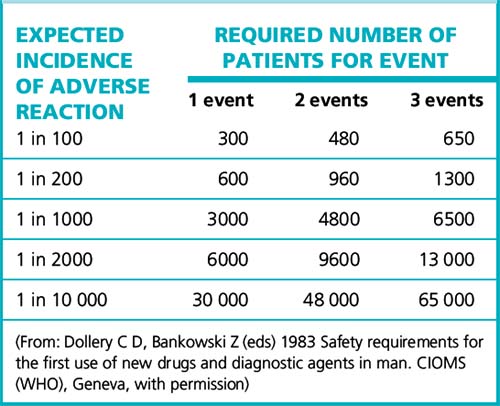
Practicalities of detecting rare adverse reactions
For reactions with no background incidence, the number of patients required to give a good (95%) chance of detecting the effect appears in Table 9.1. Assuming that three events are required before any regulatory or other action should be taken, it shows the large number of patients that must be monitored to detect even a relatively high-incidence adverse effect. The problem can be many orders of magnitude worse if the adverse reactions closely resemble spontaneous disease with a background incidence in the population.
Pharmacovigilance and pharmacoepidemiology
The principal methods of collecting data on ADRs (pharmacovigilance) are:
• Experimental studies, i.e. formal therapeutic trials of Phases 1–3. These provide reliable data on only the commoner events as they involve relatively small numbers of patients (hundreds); they detect an incidence of up to about 1 in 200.
• Observational studies, where the drug is observed epidemiologically under conditions of normal use in the community, i.e. pharmacoepidemiology and pharmacovigilance. Techniques used for post-marketing (Phase 4) studies include the observational cohort study and the case–control study. The surveillance systems are described on pages 52–53.
Drug-induced illness
The discovery of drug-induced illness can be analysed as follows4:
• A drug commonly induces an otherwise rare illness: this effect is likely to be discovered by clinical observation in the licensing (pre-marketing) formal therapeutic trials and the drug will almost always be abandoned; but some patients are normally excluded from such trials, e.g. pregnant women, and detection will then occur later.
• A drug rarely or uncommonly induces an otherwise common illness: this effect is likely to remain undiscovered. Cardiovascular risk from coxibs (e.g. rofecoxib, Vioxx) approximates as an example, but the degree of increased risk did become apparent after meta-analysis of several clinical trials and observational studies.
• A drug rarely induces an otherwise rare illness: this effect is likely to remain undiscovered before the drug is released for general prescribing. The effect could be detected by informal clinical observation or during any special post-registration surveillance and confirmed by a case–control study (see p. 52); aplastic anaemia with chloramphenicol5 and the oculomucocutaneous syndrome with practolol were uncovered in this way.
• A drug commonly induces an otherwise common illness: this effect will not be discovered by informal clinical observation. If very common, it may be discovered in formal therapeutic trials and in case–control studies, but if only moderately common it may require observational cohort studies, e.g. pro-arrhythmic effects of anti-arrhythmic drugs.
• Drug adverse effects and illness incidence in an intermediate range: both case–control and cohort studies may be needed.
Some impression of the features of drug-induced illness can be gained from the following statistics:
• In a large UK study, the prevalence of ADRs as a cause of admission to hospital was 6.5%, with a median bed stay of 8 days (4% of hospital bed capacity); most reactions were definitely or possibly avoidable; the commonest drugs were: low-dose aspirin, diuretics, warfarin, non-steroidal anti-inflammatory drugs (other than aspirin); the commonest adverse reaction was gastrointestinal bleeding.6
• Overall incidence in hospital inpatients is 10–20%, with possible prolongation of hospital stay in 2–10% of patients in acute medical wards.
• ADRs cause 2–3% of consultations in general practice.
• A study of 661 ambulatory patients found that 25% experienced adverse events, of which 13% were serious and 11% were preventable.7
• Predisposing factors for ADRs are: age over 60 years or under 1 month, female sex, previous history of adverse reaction, hepatic or renal disease, number of medications taken.
• A review of records of coroner’s inquests for a (UK) district with a population of 1.19 million during the period 1986–1991 found that, of 3277 inquests on deaths, 10 were due to errors of prescribing and 36 were caused by adverse drug reactions.8 Nevertheless, 17 doctors in the UK were charged with manslaughter in the 1990s, compared with two in each of the preceding decades, a reflection of ‘a greater readiness to call the police or to prosecute’.9
Sir Anthony Carlisle,10 in the first half of the 19th century, said that ‘medicine is an art founded on conjecture and improved by murder’. Although medicine has advanced rapidly, there is still a ring of truth in that statement, as witness anyone who follows the introduction of new drugs and observes how, after the early enthusiasm, there follow reports of serious toxic effects, and withdrawal of the drug may then follow. The challenge is to find and avoid these, and, indeed, the present systems for detecting adverse reactions came into being largely in the wake of the thalidomide, practolol and benoxaprofen disasters (see p. 63); they are now an increasingly sophisticated and effective part of medicines development.
Drugs and skilled tasks
Many medicines affect performance, and it is relevant to review here some examples with their mechanisms of action. As might be expected, centrally acting and psychotropic drugs are prominent, e.g. the sedative antidepressants, benzodiazepines, non-benzodiazepine and other hypnotics, and antipsychotics (the ‘classical’ type more so than the ‘atypicals’; see p. 322). Many drugs possess anticholinergic activity either directly (atropine, oxybutynin) or indirectly (tricyclic antidepressants, antipsychotics), the central effects of which cause confusion and impaired ability to process information. The first-generation H1-receptor antihistamines (chlorphenamine, diphenhydramine) are notably sedating and impair alertness and concentration, which features the recipient may not recognise. Drugs may also affect performance through cerebral depression (antiepileptics, opioids), hypoglycaemia (antidiabetics) and hypotension (antihypertensives). For alcohol and cannabis, see pp. 142 and 155.
Car driving is a complex multifunction task that includes: visual search and recognition, vigilance, information processing under variable demand, decision-making and risk-taking, and sensorimotor control. It is plain that prescribers have a major responsibility here, both to warn patients and, in the case of those who need to drive for their work, to choose medicines with a minimal liability to cause impairment.11 Patients who must drive when taking a drug of known risk, e.g. benzodiazepine, should be specially warned of times of peak impairment.12
Sources of adverse drug reactions
• The patient may be predisposed to an ADR by age, sex, genetic constitution, known tendency to allergy, disease of drug eliminating organs (see Ch. 8), or social habits, e.g. use of tobacco, alcohol, other recreational drugs (see Ch. 11).
• The known nature of the drug may forewarn. Some drugs, e.g. digoxin, have steep dose–response curves and small increments of dose are more likely to induce adverse or toxic reactions (see p. 92). The capacity of the body to eliminate certain drugs, e.g. phenytoin, may saturate within the therapeutic dose range so that standard increases cause a disproportionate rise in plasma concentration, risking toxic effects (see p. 356). Some drugs, e.g. antimicrobials and particularly penicillins, have a tendency to cause allergy. Anticancer agents warrant special care as they are by their nature cytotoxic (see Ch. 31). Use of these and other drugs may raise longer-term issues of mutagenicity, carcinogenicity and teratogenicity. Ingredients of a formulation, rather than the active drug, may also cause adverse reactions. Examples include the high sodium content of some antacids, and colouring and flavouring agents. The latter are designated in the list of contents by E numbers; tartrazine (E102) may cause allergic reactions.
• The prescriber needs to be aware that adverse reactions may occur after a drug has been used for a long time, at a critical phase in pregnancy, is abruptly discontinued (see p. 99) or given with other drugs (see Drug interactions, Ch. 8).
Aspects of the above appear throughout the book as is indicated. Selected topics are:
Age
The very old and the very young are liable to be intolerant of many drugs, largely because the mechanisms for disposing of them in the body are less efficient. The young are not simply ‘small adults’ and ‘respect for their pharmacokinetic variability should be added to the list of our senior citizens’ rights’.13 Multiple drug therapy is commonly found in the old, which further predisposes to adverse effects (see Prescribing for the elderly, p. 105).
Genetic constitution
Inherited factors that influence response to drugs appear in general under Pharmacogenomics (see p. 101). For convenience, we describe here the porphyrias,14 a specific group of disorders for which careful prescribing in a subgroup, the acute porphyrias, is vital.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree