(1)
Department of Pathology, University of Texas MD Anderson Cancer Center, Houston, TX, USA
Keywords
Salivary glandEpithelial tumorMesenchymal tumorPathologyImmunohistochemistryMolecular geneticsIntroduction To Salivary Gland Tumors
♦
Salivary gland tumors are predominantly epithelial (Tables 23.1, 23.2, 23.3, 23.4, 23.5 and 23.6). Mesenchymal tumors most often involve glands by extension rather than arising within the gland. The latter are also found predominantly in children and adolescents. The most commonly encountered tumors in surgical pathology will be highlighted in this chapter. The nonpleomorphic salivary adenomas can be placed under the domain of monomorphic adenoma . These, in turn, can be divided into basaloid and nonbasaloid tumors (Fig. 23.1 and Tables 23.7 and 23.8). There is also an inverse relationship between the site of the affected salivary gland and the frequency of malignancy (Table 23.9)
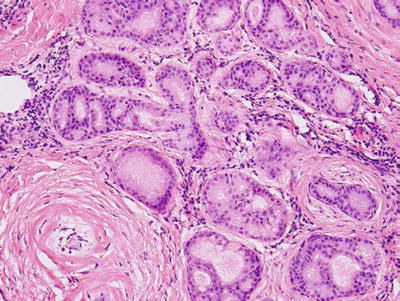
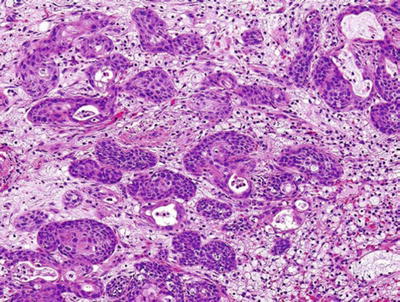
Table 23.1.
Categorical Classification of Salivary Gland Lesions
Reactive/developmental | Primary neoplasms | Metastasis |
|---|---|---|
Tumorlike | Benign | Epithelial |
Cysts | Malignant | Nonepithelial |
Table 23.2.
Tumorlike and Cystic Lesions
Tumorlike | Cystic lesions |
|---|---|
Sialolithiasis | Benign cysts |
Sialadenitis | Cystic neoplasms |
Sialadenosis | Inflammatory, specific, and nonspecific |
Ductal adenoma/hamartoma | |
Oncocytosis | |
Necrotizing sialometaplasia | |
Benign lymphoepithelial lesions and Sjögren’s syndrome | |
Chronic sclerosing sialadenitis of the submandibular gland (Küttner tumor) | |
Cystic lymphoid hyperplasia in AIDS | |
Inflammatory pseudotumor | |
Others: nodular fasciitis, sinus histiocytosis with massive lymphadenopathy (Rosai–Dorfman disease), Wegener granulomatosis |
Table 23.3.
Incidence and Location of Nonneoplastic Salivary Gland Cysts (Batsakis and Raymond 1989)
Type | Site | Frequency (%) |
|---|---|---|
Mucocele | Minor glands | 76.0 |
Salivary duct | Parotid | 9.0 |
Lymphoepithelial | Parotid and oral cavity | 7.0 |
Ranula | Sublingual gland | 6.0 |
Congenital sialectasis | Parotid | 1.5 |
Polycystic (dysgenetic) | Parotid | <1.0 |
Table 23.4.
Frequency of Retention Mucocele by Site (Modified from S. Seifert)
Site | Frequency (%) |
|---|---|
Lower lip | 23.5 |
Cheek | 13.2 |
Floor of the mouth | 12.6 |
Upper lip | 15.4 |
Palate | 8.8 |
Tongue | 4.4 |
Other areas | 16.1 |
No information | 5.9 |
Table 23.5.
Salivary Gland Neoplasms
Benign | Malignant | ||
|---|---|---|---|
Epithelial | Mesenchymal | Epithelial | Nonepithelial |
Pleomorphic adenoma (benign mixed tumor) | Angioma | Mucoepidermoid carcinoma | Sarcomas |
Basal cell adenoma/canalicular adenoma | Sialolipoma | Adenoid cystic carcinoma | Lymphomas |
Warthin tumor | Fibrous histiocytoma | Acinic cell carcinoma | |
Oncocytoma | Neurofibroma and neurilemmoma | Terminal duct carcinoma (polymorphous low-grade adenocarcinoma) | |
Myoepithelioma | Epithelial–myoepithelial carcinoma | ||
Sebaceous adenoma | Salivary duct carcinoma | ||
Ductal papilloma | Basal cell adenocarcinoma | ||
Cystadenoma | Sebaceous carcinoma | ||
Oncocytic carcinoma | |||
Adenocarcinoma, NOS (not otherwise classified) | |||
Squamous cell carcinoma | |||
Carcinoma ex pleomorphic adenoma | |||
Carcinosarcoma (sarcomatoid carcinoma) | |||
Myoepithelial carcinoma | |||
Undifferentiated carcinoma (small and large cells) | |||
Lymphoepithelioma (undifferentiated carcinoma with lymphoid stroma) | |||
Table 23.6.
Incidence of Primary Epithelial Salivary Gland Neoplasms in Different Sites (%)
Diagnosis | Parotid | Submandibular | Sublingual | Minor |
|---|---|---|---|---|
Benign | ||||
Pleomorphic adenoma | 63.3 | 59.5 | 0 | 42.9 |
Adenolymphoma | 14.0 | 0.8 | 0 | 0 |
Monomorphic adenoma | 8.0 | 2.3 | 14.0 | 11.0 |
Malignant | ||||
Mucoepidermoid carcinoma | 1.5 | 1.6 | 0 | 8.9 |
Acinic cell carcinoma | 2.5 | 0.4 | 0 | 1.8 |
Adenoid cystic carcinoma | 2.0 | 16.8 | 28.6 | 13.1 |
Adenocarcinoma (NOS) | 2.6 | 5.0 | 14.2 | 12.2 |
Squamous carcinoma | 1.1 | 1.9 | 0 | 1.2 |
Undifferentiated carcinoma | 1.8 | 3.9 | 14.2 | 2.1 |
Carcinoma ex PA | 3.2 | 7.8 | 28.6 | 7.1 |

Fig. 23.1.
Ductal adenoma.

Fig. 23.2.
Necrotizing sialometaplasia.
Table 23.7.
Types of Monomorphic Adenomas
Basal | Nonbasal |
|---|---|
Tubulotrabecular | Warthin tumor |
Solid | Oncocytoma |
Canalicular | Sebaceous lymphadenoma |
Dermal analog | Sebaceous adenoma |
Ductal adenoma (Fig. 23.1) |
Table 23.8.
Differential Characteristics Between Basal Adenomas and Canalicular Adenoma
Feature | Basal adenoma | Other types | Canalicular |
|---|---|---|---|
Sex | |||
Female/male | 1:10 | 1:1 | 1.7:1 |
Age (years) | |||
Range | 34–74 | 1–83 | 34–88 |
Mean | 58.1 | 58.6 | 65.1 |
Synchronous | 37.7% | 0 | 0 |
Dermal lesions | |||
Site (%) | |||
Parotid | 86.2 | 90.1 | 1.6 |
Submandibular | 6.8 | 4.9 | 0 |
Upper lip | 0 | 1.9 | 87.2 |
Other minor site | 6.8 | 0 | 24.7 |
Multicentricity | 48% | 0.9 | 24.0 |
Recurrences | 24% | 0 | 0 |
Malignant transformation | 28% | 3.9% | 0 |
Table 23.9.
Frequency of Epithelial Malignancy in Different Salivary Gland Sites
Site | Malignant (%) |
|---|---|
Parotid | 14.7 |
Submandibular | 37.0 |
Sublingual | 85.7 |
Minor | 46.1 |
Unknown | ? |
Tumorlike And Cystic Lesions
Tumorlike Lesions (See Table 23.2)
Sialolithiasis
♦
Predominantly in the submandibular gland
♦
Undulant and tender swelling
♦
Parotid: less frequent and difficult to diagnose clinically
Microscopic
♦
Dilated ducts
♦
Squamous metaplasia
♦
Acinar atrophy and inflammation
Sialadenitis
♦
Acute and chronic nonspecific inflammation
♦
Bacterial: Staphylococcus aureus
Viral: cytomegalovirus, paramyxovirus, Epstein–Barr virus, para- and influenza viruses, and coxsackievirus
♦
Granulomatous
Obstructive sialadenopathy
•
Ruptured mucoceles
•
Extravasated mucin
Infectious
•
Mycobacterial, cat scratch disease, tularemia, fungal, toxoplasmosis, and others
Sarcoidosis
Associated with systemic disease
•
Wegener granulomatosis, Crohn disease, and others
Associated with salivary gland tumor
•
Acinic cell carcinoma
•
Mucoepidermoid carcinoma
•
Warthin tumor
•
Lymphoepithelial lesions
Foreign body granulomas
•
Iatrogenic (sialography)
Sialadenosis
♦
Hypertrophy and hyperplasia of acini
♦
Associated with systemic and neural disorders
♦
Presents as bilateral painless and recurrent swelling
♦
Etiology: neurogenic, metabolic, or hormonal
Necrotizing Sialometaplasia (Fig. 23.2)
Clinical
♦
Benign self-limiting reactive inflammatory process
♦
Sites: palate, 81.7%; other oral sites, 7.0%; major glands, 7.8%; and other, 3.5%
♦
Age: 40–60 years
♦
More men than women
♦
Uncertain etiology, possible vascular infarction
Macroscopic
♦
Mucosal site, ulceration, and painful swelling
♦
Nonulcerated: one-third of lesions
♦
Lobular distribution
♦
Central ductal squamous metaplasia
♦
Peripheral necrotic and inflamed acini
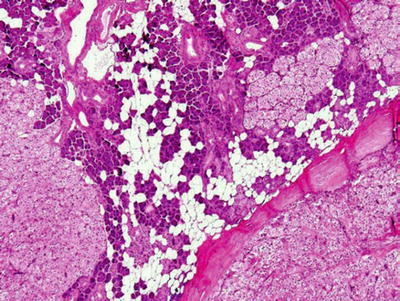
Fig. 23.3.
Oncocytosis/oncocytic hyperplasia.
Differential Diagnosis
♦
Squamous carcinoma
Infiltrative, keratinization, cellular features of malignancy
Lacks lobular preservation
♦
Mucoepidermoid carcinoma
Mucinous cells, infiltrative intermediate cells, and cystic formations
Oncocytic Hyperplasia of Ductal and Acinar Structure (Fig. 23.3)
♦
Unilateral or bilateral enlargement
♦
Preservations of lobular architecture
♦
Old age
Lymphoepithelial Lesions
Clinical
♦
Idiopathic, autoimmune disease, HIV infection
♦
Localized, systemic
♦
Older women
♦
Recurrent painful swelling
♦
Epimyoepithelial islands
♦
Focal periductal lymphoreticular proliferation
♦
Partial or total obliteration of the acinar structure
♦
Epimyoepithelial islands
♦
Marked lymphoreticular infiltrate with germinal centers
♦
Hyaline-like material deposits
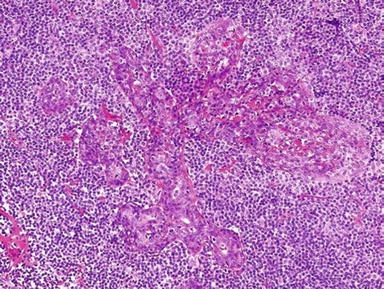
Fig. 23.4.
Lymphoepithelial lesion.
Sjögren Syndrome
Clinical
♦
Associated with
Keratoconjunctivitis sicca
Xerostomia
Connective tissue disease
♦
Assessed by labial minor salivary gland biopsy
Focus score: number of inflammatory foci/4 mm of the gland (focus is defined as ≥50 lymphocytes)
High incidence of non-Hodgkin lymphoma in women
Nonlymphoid malignancy, low frequency
Differential Diagnosis
♦
Lymphoepithelial carcinoma
♦
Lymphoma (MALT)
Both tumors may arise in this setting
Küttner Tumor (Chronic Sclerosing Sialadenitis of the Submandibular Gland)
♦
Unilateral
♦
Lymphoplasmacytic periductal infiltrate with fibrosis
♦
Clinically tumorlike
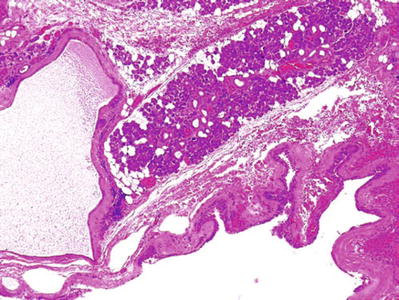
Cystic Lymphoid Hyperplasia (HIV+ Subjects) (Fig. 23.5)
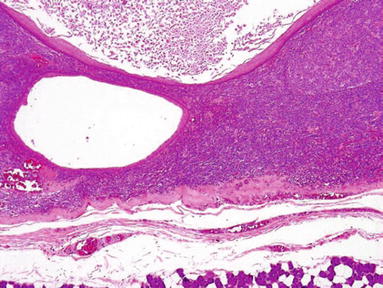
Fig. 23.5.
Cystic lymphoid hyperplasia .
Clinical
♦
HIV-infected patients
♦
Nodular or diffuse enlargement of salivary glands
♦
Unknown pathogenesis
♦
(p24) Antigen infection
Microscopic
♦
Glandular atrophy
♦
Intense lymphocytic proliferation with follicular hyperplasia
♦
Cystic formation with epithelial lining from ductal inclusion
♦
Epimyoepithelial islands
Inflammatory Pseudotumors
Clinical
♦
Firm, nodular swelling in the parotid
Microscopic
♦
Myofibroblastic proliferation and chronic inflammatory cells
Immunohistochemistry
♦
SMA, MSA, and CD68+
Salivary Gland Cysts (See Tables 23.3 and 23.4)
Benign Cysts of the Salivary Gland: An Overview
♦
Secondary acquired, pseudocysts
♦
Most secondary pseudocysts are mucocele
♦
Parotid duct cyst (11%), lymphoepithelial cyst (7%), and ranula (5%)
Cystic Neoplasms
♦
Cystic mucoepidermoid carcinoma
♦
Warthin tumor
♦
Cystadenocarcinoma
♦
Sebaceous lymphadenoma
Inflammatory, Specific, and Nonspecific
Dysgenetic Cysts
♦
Polycystic dysgenetic lesions (Fig. 23.7)
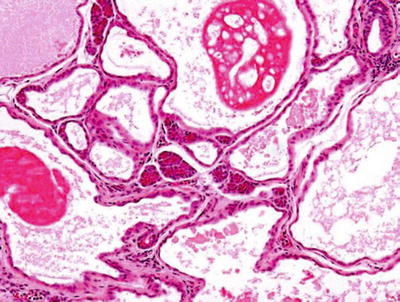

Fig. 23.7.
Dysgenetic polycystic lesion.
Rare
Mainly in the parotid
Multiple cysts lined by simple epithelial lining
Female predominance
Delayed clinical manifestation
Bilateral
Fluctuating, nontender parotid swelling
Diagnosis: sialogram, spare main salivary duct
Surgical excision for diagnosis and treatment
Etiology: most likely developmental
♦
Sclerosing polycystic adenosis (Figs. 23.8 and 23.9)
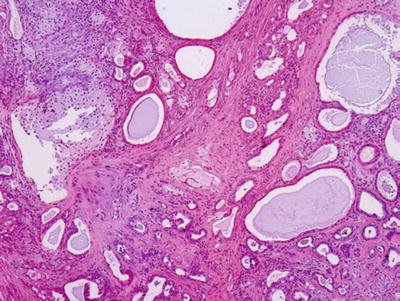
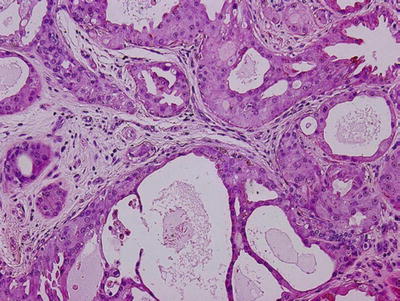

Fig. 23.8.
Sclerosing polycystic adenosis.

Fig. 23.9.
Sclerosing polycystic adenosis.
Resemblance of fibrocystic disease of the breast in its diversity
Preservation of lobular architecture
Duct ectasia, with or without epithelial hyperplasia, apocrine-like metaplasia
Occasional collagenous spherulosis
Uncapsulated but often demarcated, limitation of nodules from adjacent salivary tissue
Differential diagnosis: dysgenetic polycystic parotid gland, obstructive sialadenitis
Surgical excision for diagnosis and treatment
Etiology: unclear, pseudoneoplastic benign entity versus neoplastic
♦
Cyst of the submandibular gland
Benign cyst lined by flattened epithelium
Etiology: duct segmentation
Secondary Cysts
♦
Salivary duct cyst
Mainly in the parotid
10% of all nonneoplastic cysts
More than two-thirds male in the second decade of life
Multilayered, cuboidal epithelial lining
Occasional oncocytic and squamous metaplastic changes
Cystic contents, spheroliths or crystalline precipitate
Pathogenesis: ductal obstruction
♦
Lymphoepithelial cyst
Locations: parotid, intraparotid lymph nodes, and floor of the mouth
Lining: flattened or multilayered epithelium surrounded by lymphocytes and lymphoid follicles
Sebaceous glands and goblet cells may be present
Pathogenesis: displacement of epithelium into lymphoid tissue or proliferation of bronchial pouch epithelium
Related to benign lymphoepithelial lesions, chronic myoepithelial sialadenitis, and HIV-related cystic lymphoid hyperplasia
♦
Retention mucocele
Less common, 15%
Older age, >20 years
Site: minor salivary glands
Cyst lined by flat, cuboidal, or multilayered epithelium surrounded by thick fibrous capsule
Pathogenesis: obstruction
♦
Extravasation
Most common, 85%
Sites: lip, cheek, and floor of the mouth
More in men (60%)
Peak incidence: second decade
Initially ill-defined mucus lakes, followed by granuloma formation and muciphages, finally mucin-filled pseudocysts (no epithelial lining)
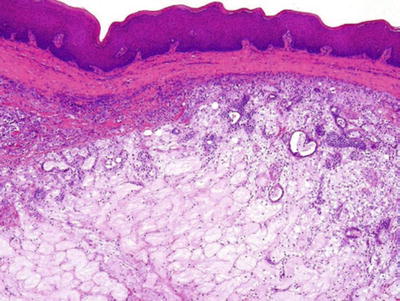
Fig. 23.10.
Subepithelial encapsulated mucin leakage (ranulas).
Benign Cystic Lesions of the Neck
♦
Thyroglossal duct cyst
Midline, two-thirds below the hyoid bone, one-third off midline, anteromedial to carotid artery and jugular vein
♦
Branchial cleft cyst
Lateral neck, unrelated to hyoid, majority near angle of mandible; if small, anterior to sternomastoid and lateral to carotid artery and internal jugular vein
♦
Parathyroid cyst
95% near inferior thyroid margin, off midline; anterior to carotid artery and internal jugular vein
♦
Cervical thymic cyst
Off midline, low neck, anterior to carotid artery and internal jugular vein
♦
Cystic hygroma
♦
Dermoid cyst
Near midline, usually in the upper neck
Benign teratoma
Usually near the thyroid gland
♦
Cervical ranula
Off midline and suprahyoid in submental and submandibular triangle
Benign Tumors (See Tables 23.5 And 23.6)
Epithelial
Pleomorphic Adenoma (Mixed Tumor)
Clinical
♦
Comprised of 55–70% of all salivary neoplasms
♦
More in females in the fourth decade
♦
Painless, slow-growing mass
♦
In the parotid: 90% superficial lobe, 10% deep lobe
Macroscopic
♦
Well-defined lobulated mass
♦
Multifocal in 0.5% of cases
♦
Variegated appearance, cartilaginous
♦
Biphasic epithelial and mesenchymal elements
♦
Epithelial cells
Tubules, nests, ribbons, and files with occasional squamous, oncocytic, or sebaceous differentiation
♦
Myoepithelial cells
Spindle or plasmacytoid cells
♦
Stroma
Mucoid, myxoid, hyaline, or chondroid
Rarely adipose tissue and bone
♦
Crystals
Tyrosine-calcium oxalate , collagenous spherules
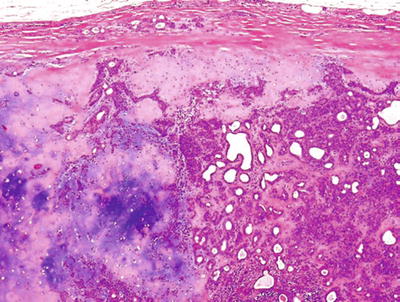
Fig. 23.11.
Pleomorphic adenoma cartilaginous and ductal components.
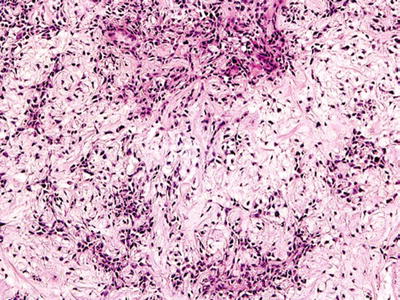
Fig. 23.12.
Pleomorphic adenoma (myxoid stroma).
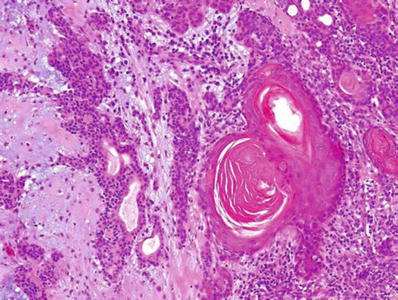
Fig. 23.13.
Pleomorphic adenoma (squamous differentiation).
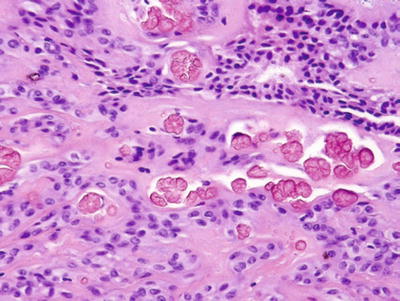
Fig. 23.14.
Pleomorphic adenoma with crystals.
Immunohistochemistry
♦
Generally noncontributory
♦
S-100 protein and smooth muscle actin (SMA)+in stroma and myoepithelial cells
Cytogenetics and Molecular Genetics
♦
Alteration, rearrangements, and LOH at 8q21 and 12q 14–15 regions
Differential Diagnosis
♦
Adenoid cystic carcinoma
Lacks mesenchymal elements; clear demarcation between collagenous, epithelial, and mucoid materials
Propensity for perineural invasion
♦
Myoepithelioma
Lacks ductal and mesenchymal elements
♦
Terminal duct carcinoma
Variegated epithelial patterns
Lacks mesenchymal features
Recurrent Mixed Tumor
♦
Histologically similar to original tumor
♦
Multiple nodules
♦
Extension to surrounding tissue
Myoepithelioma
Clinical
♦
Uncommon, 1.5% of all salivary tumors
♦
2.2–5.7% of all benign tumors
♦
Male to female ratio, 1:1
♦
Most common sites: palate and parotid
Macroscopic
♦
Well-demarcated mass
♦
Tan, homogenous, and solid
♦
Comprised of >90% myoepithelial cells
♦
Cellular, mucoid, or hyalinized stroma may be present
♦
Lacks ductal differentiation
♦
Cell types: spindle, plasmacytoid, epithelioid, and clear cells
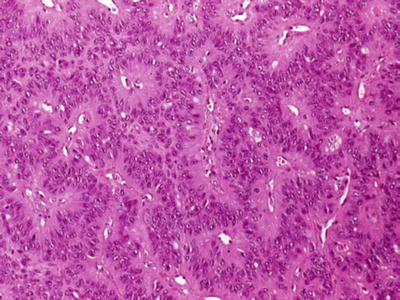
Fig. 23.15.
Myoepithelioma (organoid pattern).
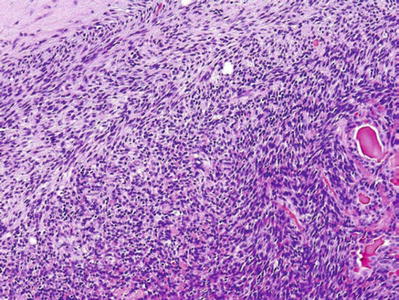
Fig. 23.16.
Myoepithelioma (spindle cell).
Immunohistochemistry
♦
Keratin (CK14)+, SMA+, S-100 protein+
Electron Microscopy
♦
Microfilaments, 50–100 Å in size
♦
Pinocytic vesicles
Differential Diagnosis
♦
Spindle cell myoepithelioma
Leiomyoma, nerve sheath tumors, synovial sarcoma, nodular fasciitis
Keratin immunoreactivity excludes mesenchymal tumors
♦
Plasmacytoid myoepithelioma
Plasmacytoma, positive for monoclonal light chain reaction and negative keratin−
♦
Clear cell myoepithelioma
Metastatic renal cell carcinoma : vascular, cellular atypia, hemorrhage, history of RCC
Myoepithelial carcinoma : cellular atypia, infiltrative growth
Oncocytoma
Clinical
♦
Rare, 1% of all salivary tumors
♦
Women slightly more than men
♦
Bilaterality, in 7% of cases
♦
Main presentation, swelling
Macroscopic
♦
Solitary, encapsulated
♦
Reddish brown, smooth, and homogenous cut surface
♦
Large polygonal eosinophilic cells with central round nuclei
♦
Cord, ribbon, and acinar formation
♦
Focal mucinous or squamous metaplasia may be present
♦
Clear cell variant (clear cell oncocytoma)
♦
Oncocytic hyperplasia may occasionally be found at periphery
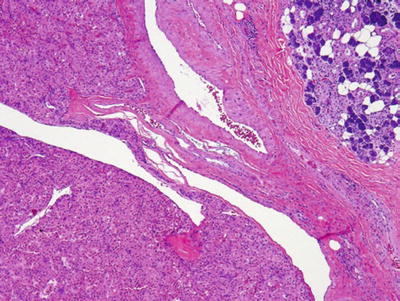
Fig. 23.17.
Oncocytoma.
Immunohistochemistry
♦
AMA (antimitochondrial antibody)
Electron Microscopy
♦
Large accumulation of mitochondria with lamellar structure
♦
Occasionally large glycogen particles
Special Stains
♦
Phosphotungstic acid hematoxylin (PTAH)+ stain
Differential Diagnosis
♦
Conventional oncocytoma
Pleomorphic adenoma with oncocytic metaplasia: features of pleomorphic adenoma
Mucoepidermoid carcinoma: PTAH–, mucinous, intermediate, and squamous differentiation
Acinic cell carcinoma: acinar differentiation, PTAH–, granules
Oncocytosis and oncocytic hyperplasia: diffuse, lacks fibrous encapsulation, retains architecture
♦
Clear cell oncocytoma
Epimyoepithelial carcinoma: dual cellular differentiation (myoepithelial and ductal)
Metastatic renal cell carcinoma: high vascularity, history of renal cell carcinoma
Warthin Tumor (Adenolymphoma)
Clinical
♦
Second most common benign tumor
♦
Superficial, slow-growing mass
♦
Exclusively the parotid, 2–15% of all parotid tumors
♦
Bilaterality not uncommon
♦
Male to female ratio, 8:1
Macroscopic
♦
Well circumscribed and cystic
♦
Brownish, creamy materials
♦
Oncocytic epithelial lined spaces with occasional papillary formation
♦
Lymphoid stroma, with germinal centers
♦
Occasional goblet, squamous, and sebaceous cells
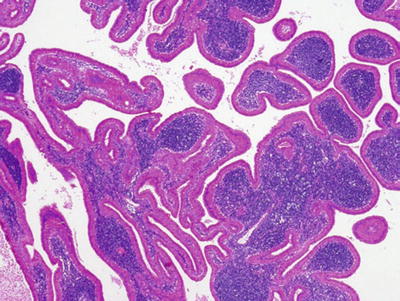
Fig. 23.18.
Warthin tumor oncocytic ductal proliferation with lymphoid stroma.
Immunohistochemistry
♦
Noncontributory
Electron Microscopy
♦
Enlarged mitochondria in oncocytes
Cytogenetics
♦
Structural alteration in chromosome 7
♦
A subset of WT t(11;19)
Differential Diagnosis
♦
Oncocytoma (in stroma-deficient Warthin tumor)
Lacks cystic formation
♦
Sebaceous adenoma, squamous carcinoma, and mucoepidermoid carcinoma
Lack lymphoid stroma
Minimal oncocytic features
Sebaceous Lymphadenoma
Clinical
♦
Rare, 0.1% of all adenomas
♦
The parotid is the most common site
♦
Male to female ratio, 1:1
♦
Painless mass
Macroscopic
♦
Circumscribed and encapsulated mass
♦
Solid sebaceous or cystic nests and metaplastic ducts in lymphoid stroma
♦
Giant cell reaction to extravasated cystic content
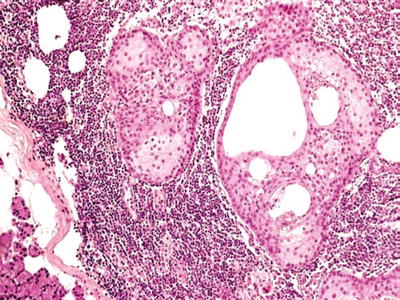
Fig. 23.19.
Sebaceous lymphadenoma.
Sebaceous Adenoma
Clinical
♦
Rare, 0.1% of all salivary gland tumors
♦
Males slightly more than females
♦
Parotid, submandibular, and oral cavity glands are the most common sites
Macroscopic
♦
Solid, gray, well-circumscribed mass
Microscopic
♦
Benign irregular sebaceous cellular nests in lymphoid stroma
♦
Large cystic formation
Basal Cell Adenoma (see Table 23.7)
Clinical
♦
1–3% of all major salivary gland tumors
♦
Mostly in women
♦
The parotid is the most common site
♦
May present within the cervical lymph node
Macroscopic
♦
Encapsulated, round, or oval mass
♦
Grayish or white homogenous cut surface
♦
Average size 2 cm
♦
Occasional cystic formation
♦




Trabecular, solid , mixed, and membranous cellular patterns
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree