Treatment of Splenic Artery Aneurysms
Gilbert R. Upchurch Jr
Frank C. Vandy
John E. Rectenwald
James C. Stanley
The optimal surgical treatment of splenic artery aneurysms (SAAs) is dependent on recognition of the disease’s underlying pathology, its clinical relevance, and choosing an appropriate therapeutic intervention. Endovascular treatment of SAA has become increasingly important as catheter-based therapies have been incorporated into the surgeon’s armamentarium.
Splenic Artery Aneurysms
SAAs are the most common splanchnic arterial aneurysm, accounting for approximately 60% of all true splanchnic aneurysms. Women are affected four times more frequently than men. SAAs typically are saccular and occur at arterial bifurcations. They are multiple in approximately 20% of cases.
Three known factors are associated with the development of SAAs. The first factor relates to the effects of repeated pregnancy on the vessel wall. Approximately 40% of women with SAAs are grand multiparas, having completed six or more pregnancies. Aneurysm formation in these patients may be due to hormonal effects on elastic tissue and the marked increase in splenic artery
blood flow occurring with pregnancy. The second factor is systemic arterial dysplasia. This receives support from the observation that 2% of patients having renal artery fibrodysplasia also have SAA. The third factor associated with SAA formation is the presence of portal hypertension, with 10% to 15% of these patients exhibiting these aneurysms. Both hyperdynamic flow and hormonal consequences of end-stage cirrhosis cause increased splenic artery diameters in portal hypertension and may result in SAA.
blood flow occurring with pregnancy. The second factor is systemic arterial dysplasia. This receives support from the observation that 2% of patients having renal artery fibrodysplasia also have SAA. The third factor associated with SAA formation is the presence of portal hypertension, with 10% to 15% of these patients exhibiting these aneurysms. Both hyperdynamic flow and hormonal consequences of end-stage cirrhosis cause increased splenic artery diameters in portal hypertension and may result in SAA.
Although many SAAs exhibit extensive calcific arteriosclerosis, this is considered a secondary event rather than the primary cause of most of these aneurysms. Additional causes of SAAs and pseudoaneurysms include trauma, as well as inflammatory diseases, particularly pancreatitis with associated pseudocyst formation.
SAAs are most often asymptomatic. Vague epigastric or left upper quadrant discomfort is a nonspecific symptom attributed to these lesions. Roentgenographic demonstration of left upper quadrant, curvilinear, signet ring-like calcification may reveal the presence of an SAA (Fig. 1). However, most are recognized as incidental findings during imaging studies, including computed tomographic (CT) angiography (CTA), magnetic resonance angiography (MRA), and arteriography for unrelated diseases.
Rupture of SAA occurs in less than 2% of patients. SAA rupture in nonpregnant patients is fatal in less than 25%. Rupture has been reported in more than 90% of aneurysms recognized during pregnancy, with a maternal mortality approaching 75% and fetal mortality exceeding 95%. However, many unruptured SAAs exist during pregnancy and go unrecognized clinically. Rupture often presents with hemorrhage contained within the lesser sac. Hemodynamic collapse occurs as blood escapes through the foramen of Winslow. Lesser sac tamponade that postpones catastrophic intraperitoneal bleeding accounts for the so-called double-rupture presentation.
Surgical treatment of SAA is warranted in all patients with symptomatic aneurysms, as well as in pregnant patients and women of childbearing age who might conceive at a later time. Elective treatment, by means of either open surgical procedure or endoluminal approach, should be considered for asymptomatic SAAs 2 cm or greater in diameter. Importantly, percutaneous SAA embolization provides a therapeutic option for the historically poor operative candidates. Given the amount of radiation involved with endoluminal treatment of SAAs, open surgical aneurysmectomy or aneurysm exclusion is preferred in pregnant patients, especially those in the first trimester. Open repair is recommended only when percutaneous treatment has failed or is not appropriate for that patient, and in those circumstances operative mortality should be less than 0.5% to justify its performance.
Endovascular Treatment of Ssas
The goals of endovascular therapy are the same as for open surgery: exclusion of the aneurysm from circulation and prevention of rupture. Major advantages of endovascular therapy over open surgical therapy include shorter length of hospital stay, shorter recovery time, and avoidance of general anesthesia. In high-risk patients, endovascular therapy is the safest option.
Endovascular treatment of SAA has certain limitations. Use of iodinated contrast in patients with allergies to such agents or in patients with renal failure may prevent catheter-based therapy from being an option. Use of alternative contrast agents, such as carbon dioxide, may be possible in such cases. Splenic infarction following aneurysm embolization is also a concern. Postembolization syndrome, defined as a combination of fever, abdominal pain, slowed gastrointestinal transit, pleural effusion, and possibly pancreatitis, has been shown to occur in 15% to 30% of patients undergoing SAA embolization. Asymptomatic infarction or atrophy may occur in up to 40% of patients.
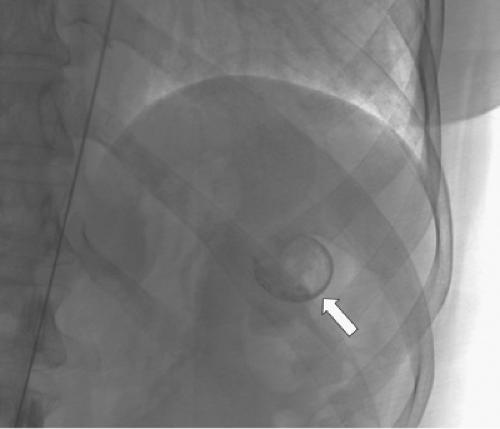
SAA embolization is usually performed after percutaneous femoral access is obtained. Occasionally, limited groin access may necessitate a brachial approach and because of the use of larger sheath sizes, an open surgical exposure of the brachial artery may be required. Catheterization of the splenic artery can usually be performed without formal aortography using selective catheters. Following cannulation of the celiac artery, the splenic artery may be identified with contrast injections or simply the tortuous and looped path of the guidewire (Fig. 2). The serpiginous path of the splenic artery can make distal hilar access difficult and use of a microcatheter is appropriate in this setting. The degree of tortuosity and the location of the aneurysm govern the mode of SAA embolization.
Proximal aneurysms without significant tortuosity may be successfully treated with deployment of a vascular occlusion plug proximal and distal to the aneurysm (Figs. 3 and 4). Alternatively, a covered stent graft may be placed across the aneurysm. However, both covered stent grafts and large vascular plugs are limited by the difficulty with which these stiff devices navigate the curves of the splenic artery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree