Treatment of Major Hepatic Trauma
Donald D. Trunkey
Prior to 1965, the morality from liver injuries that were diagnosed and operated on was very high (Table 1). When the operation was done, treatment consisted of sutures and packs, and some surgeons advocated routine drainage. The cautery was very controversial and continued to be controversial until World War II. Based on the work by Madding and Kennedy, treatment changed during World War II, and resection became part of the surgeon’s armamentarium. This led to a remarkable reduction in overall mortality. After shifting to this management, 829 wounds to the liver were seen in an 18-month period, and of these patients, 53 died.
The year 1965 was pivotal with the introduction of diagnostic peritoneal lavage. Previously undiagnosed liver injuries were now possible to diagnose. The great majority of these were grade I to III liver injuries, and, thus, it can be appreciated that surgeons had been doing nonoperative management of liver injuries for years; they just didn’t know it. Evidence for this is present in at least three papers. In a study from Canada, 35% of patients lacked any clinical signs of blunt abdominal trauma with injuries to the spleen or liver. Similarly, a study from Michigan showed that out of 87 patients subsequently diagnosed by autopsy, 45% had undiagnosed liver injuries. This was affirmed by a study published in the Surgical Clinics of North America in 1972.
One of the problems with diagnostic peritoneal lavage and better diagnosis of liver injuries is that in 30% of patients this leads to a nontherapeutic celiotomy. The year 1981 turned out to be another pivotal year with the introduction of computed tomography (CT) scanning to make the diagnosis of intra-abdominal solid organ injury. With this diagnostic modality, it was now possible for the surgeon not only to make the diagnosis, but also to make a rational decision as to whether the patient needed operation based on the degree of injury and hemodynamic stability. More recent studies now show that most patients with grade I and II injuries can be managed nonoperatively, and patients with grade III, IV, and V injuries will require operation in 50% to 75% of the cases (Algorithms 1 and 2; Figs. 1 and 2).
Since World War II, there have been two significant events that have changed the approach to major hepatic trauma: pioneering work in the anatomy of the liver and new operative techniques for elective hepatic resection. We now have a better understanding of segmental anatomy, which has allowed segmental resection, and this has led to a marked increase in elective resection of tumors and obstruction of the porta hepatis. The second factor influencing liver surgery has been liver transplant operations, which has furthered our understanding of the anatomy and has particularly influenced surgeons in their ability to perform hepatic isolation procedures.
Repair and resection for treatment of hepatic trauma demand a working knowledge of the anatomy of the liver. The segmental approach to liver anatomy that is presented here is valuable for the surgeon for prediction of the locations of vascular structures within the liver substance. Whereas small segmental resections are feasible in elective surgery, liver resection for trauma is almost exclusively restricted to nonanatomic debridement of nonviable liver tissue, left lateral segmentectomy, and formal right or left hepatic lobectomy.
Table 1 Mortality in Rupture of Liver, 1872 to 1961 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
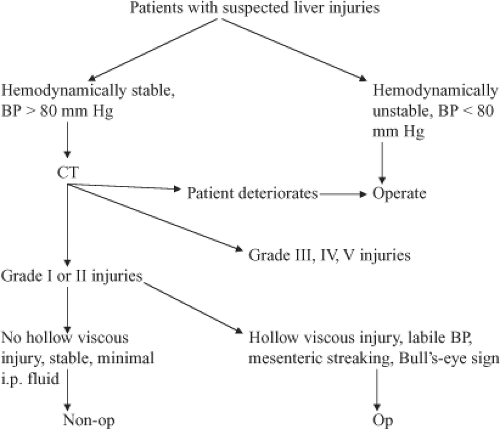 Algorithm 1. Algorithm for evaluating patients with suspected liver injuries. BP, blood pressure; CT, computed tomography. |
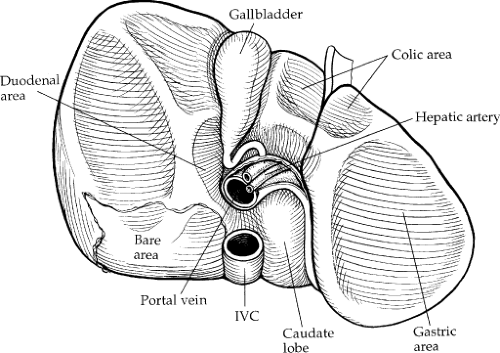
The liver has three major surfaces: superior, inferior, and posterior. The superior and posterior surfaces rest against the diaphragm; however, the inferior surface is more complex, containing the gallbladder, hepatic ducts, hepatic arteries, and portal veins (Fig. 3). The liver is attached to surrounding structures by ligaments, the reflection of the parietal peritoneum (Fig. 4). The coronary ligament is formed along the superior surface of the right lobe and secures the liver to the diaphragm posteriorly and superiorly. The two leaves of the coronary ligament join at the extreme left to form the triangular ligament. The
falciform ligament and ligamentum teres (obliterated umbilical vein) provide anterior attachment of the midportion of the liver to the anterior abdominal wall.
falciform ligament and ligamentum teres (obliterated umbilical vein) provide anterior attachment of the midportion of the liver to the anterior abdominal wall.
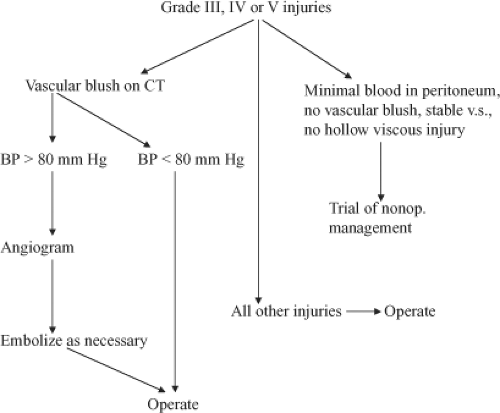 Algorithm 2. Algorithm for evaluating patients with grade III, IV, or V injuries. BP, blood pressure; CT, computed tomography. |
Morphologically, the liver is described as two main (right and left) and two accessory (quadrate and caudate) lobes. The right and left lobes are divided by the major fissure, with the right being larger. The quadrate lobe is the portion of the right lobe that lies anterior to the transverse hilar fissure, medial to the gallbladder fossa, and lateral to the umbilical fissure. The caudate lobe lies posterior to the transverse liver fissure.
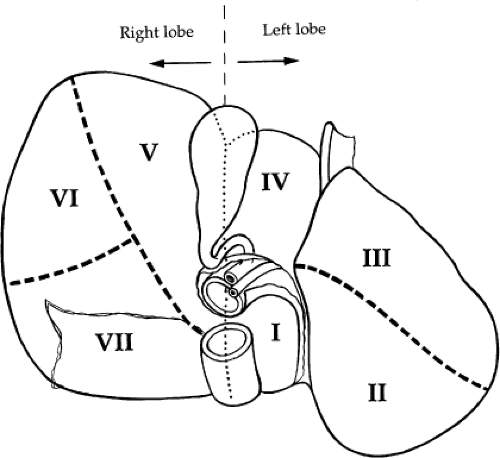
Surgical treatment of traumatic injuries relies on a functional anatomic description of the liver. Segmental and anatomic resection has been well documented by Bismuth using an anatomic description from Couinaud. Hepatic segmentation based on the distribution of the portal pedicle and location of hepatic veins defines the functional anatomy (Figs. 3 and 4). The three main hepatic veins (i.e., the right, left, and middle) divide the liver into four sections: right posterior lateral, right anterior medial, left anterior, and posterior. Each of these sectors receives a portal pedicle. Sectors of the right liver are further divided into two segments each: the anterior medial sector, segment IV anteriorly and segment VIII posteriorly; and the posterior lateral sector, segment VI anteriorly and segment VII posteriorly. The left anterior sector is divided by the umbilical fissure into segment IV, the anterior portion of the quadrate lobe, and segment III, the anterior portion of the left lobe of the liver. The posterior sector is composed of only one segment (II) and lies in the posterior portion of the left lobe. The caudate lobe is considered independently as segment I.
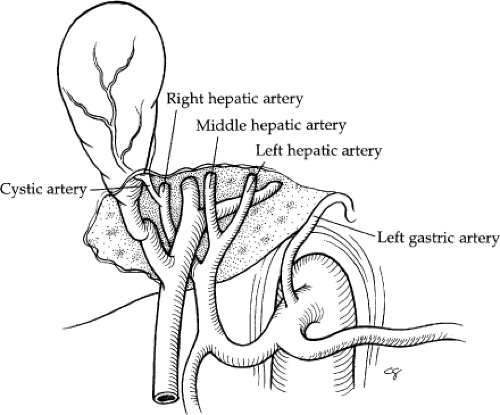
The hepatic artery supplies 25% of the blood flow to the liver and 50% of the oxygen. In 55% of cases, the hepatic artery supply is exclusive from the celiac trunk (Fig. 5). The common hepatic artery is the contribution of the celiac trunk after the left gastric and splenic arterial branches. The common hepatic artery traverses along the upper border of the head of the pancreas, and then it turns to ascend in the lesser omentum. It lies to the left of the common bile duct and anterior to the portal vein. The gastroduodenal artery arises from the distal horizontal position of the common hepatic artery. The right gastric artery arises from the proper hepatic (40% of cases) or left hepatic artery (40% of cases).
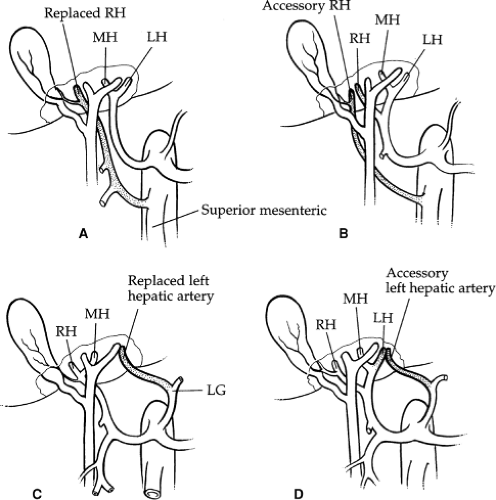
The hepatic artery bifurcates in the porta hepatis to give rise to the right and left hepatic arteries. The right hepatic artery courses to the right behind the common bile duct. The anterior and posterior segmental arteries of the right lobe take separate origin from the right hepatic artery. The anterior segmental branch courses along the gallbladder fossa in close proximity to the cystic duct. The left hepatic artery runs upward obliquely to the left and divides into its two terminal branches, the medial and lateral segmental arteries. The medial segmental artery descends into the quadrate lobe (segment IV). The lateral segmental artery travels obliquely toward the upper, outer aspect of the lateral segment, where it divides into the superior and inferior branches. The blood supply to the caudate lobe (segment I) is variable. In approximately 35% of cases, the entire blood supply of the caudate lobe comes from the right hepatic artery, and, in 12%, it comes entirely from the left hepatic artery. In the majority, however, the blood supply is from both. Aberrant hepatic arteries occur frequently in 40% to 50% of cases. The most frequent anomaly is the left hepatic artery, which arises from the gastric artery (25% to 30%) (Fig. 6A, B). This includes a 10% incidence of a totally replaced left hepatic artery and a 15% incidence of an accessory left hepatic artery. The right hepatic artery originates from the superior mesenteric artery in 17% of cases: 10% total replacement and 7% accessory (Fig. 6C, D). The middle hepatic artery arises from the left or right
hepatic artery with equal frequency. The cystic artery is usually a branch of the right hepatic artery. It reaches the gallbladder behind the common hepatic duct after traversing the hepatic triangle to the right of the common hepatic duct. In 85% to 90% of cases, there is a single cystic artery.
hepatic artery with equal frequency. The cystic artery is usually a branch of the right hepatic artery. It reaches the gallbladder behind the common hepatic duct after traversing the hepatic triangle to the right of the common hepatic duct. In 85% to 90% of cases, there is a single cystic artery.
The portal vein carries 75% of the blood flow to the liver and 50% of the oxygen. The portal vein is formed by the confluence of the superior mesenteric vein and the splenic vein behind the neck of the pancreas. Approaching the liver within the porta hepatis, the portal vein lies anterior to the inferior vena cava and to the left of the common bile duct and the hepatic artery. The portal vein is 7 to 10 cm in length and bifurcates into the left and right portal veins. The portal lobar veins lie posterior to the hepatic veins and bile ducts. The right portal vein is short and divides into anterior and posterior segmental vessels. Each of these segmental vessels further divides into inferior and superior subsegmental branches. The right portal vein sends a branch to the right side of the caudate lobe (segment I).
The left portal vein is longer than the right and courses to the left in the hilar plate from the bifurcation. It then turns inferiorly in the liver at the umbilical fossa. The superior and inferior subsegmental veins of the lateral segment (segments II and III) arise from the left side of the umbilical portion of the left portal vein. The medial segmental veins rise from the right side of the umbilical portion of the left portal vein. This fact is of importance when performing a left lateral segmentectomy (segments II and III), as the umbilical portion of the left portal vein should be left intact.
Venous drainage of the liver is quite simple. The hepatic veins lie in the planes dividing the segments of the liver. There are three major veins: right, middle, and left. The right hepatic vein is the largest and drains the anterior and posterior portions of the right lobe of the liver (segments VI, VII, and VIII). The middle and left hepatic veins frequently enter the inferior vena cava as a single trunk. The middle hepatic vein drains the superior aspect of the anterior segment of the right lobe (segment V). The left hepatic vein drains the superior aspect of the medial and lateral segments of the left lobe (segments II, III, and IV). The caudate lobe (segment I) usually drains directly into the inferior vena cava via multiple small branches.
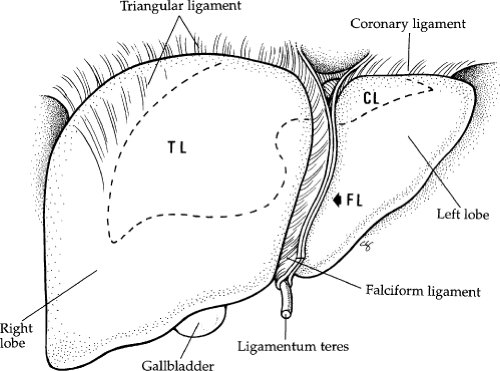 Fig. 2. Superficial anatomy of the liver showing the ligaments. CL, coronary ligament; FL, falciform ligament; TL, triangular ligament. |
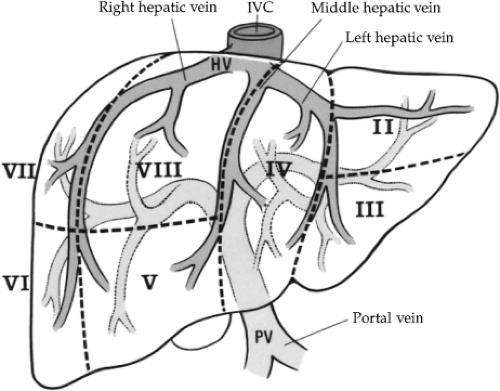 Fig. 3. An anterior view of segmental anatomy. HV, hepatic vein; IVC, inferior vena cava; PV, portal vein. |
The biliary drainage of the liver follows a segmental and lobar pattern and shares a common pathway with the blood supply (portal triad). The right hepatic duct is formed by the joining of the anterior and posterior segmental ducts of the porta hepatis. In 30% of cases, one of the two ducts, usually the posterior, crosses the segmental fissure to drain in the left hepatic duct. The left hepatic duct is formed by the confluence of the medial and lateral segmental ducts. This duct is in the left segmental fissure in 50% of cases and to the right of the fissure in 42%. In the caudate lobe (segment I), drainage is variable and may flow into either the left or right duct. The common hepatic duct is formed by the confluence of the right and left hepatic ducts in the transverse fissure of the liver. Its distal end is defined by the junction of the cystic duct and may range from 1.0 to 7.5 cm in length. The normal diameter is 4 mm. The common bile duct is a continuation of the common hepatic duct, distal to the cystic duct. It ranges in length from 2 to 7 cm. It has a consistent course and is divided into four portions: supraduodenal, retroduodenal, pancreatic, and intramural. The supraduodenal lies between layers of the hepatic duodenal ligament, anterior to the foramen of Winslow. The retroduodenal portion is between the superior margin of the first portion of the
duodenum and the superior edge of the pancreas. The gastroduodenal artery lies to the left, and the posterosuperior pancreatic duodenal artery lies anterior to the bile duct. The pancreatic portion of the common bile duct passes from the upper margin of the head of the pancreas obliquely to the right posterior portion of the pancreas to its entrance into the duodenum. The intramural portion of the common bile duct travels an oblique path approximately 1.5 cm long through the duodenal wall. It joins with the main pancreatic duct inferiorly.
duodenum and the superior edge of the pancreas. The gastroduodenal artery lies to the left, and the posterosuperior pancreatic duodenal artery lies anterior to the bile duct. The pancreatic portion of the common bile duct passes from the upper margin of the head of the pancreas obliquely to the right posterior portion of the pancreas to its entrance into the duodenum. The intramural portion of the common bile duct travels an oblique path approximately 1.5 cm long through the duodenal wall. It joins with the main pancreatic duct inferiorly.
The dissection of the hilar plate from the liver parenchyma was first described by Couinaud in 1957. Cholecystectomy before the incision of the peritoneal reflection and Glisson’s capsule aids the surgeon in entering the transverse fissure or porta hepatis. After the cholecystectomy, an incision is made at the junction of the peritoneal reflection of the porta hepatis to the Glisson’s capsule anteriorly in a U-shaped fashion directed toward the bifurcation of the left and right portal system and then moving toward the falciform ligament. By gently retracting the porta hepatis medially and lifting upward, a second incision in the peritoneal reflection is carried across from the gallbladder fossa over the superior margin of the caudate process. Using blunt dissection, the right porta hepatis can be encircled. After division of the gastrohepatic ligament in the bare area, the left porta hepatis can be encircled. Access to the retrohepatic cava requires lifting the right lobe anteriorly and medially after all of the ligamentous attachments have been taken down from the diaphragm. As the posterior peritoneum reflects up on to the cava, it is incised sharply with the scissors or electrocautery unit and carried cephalad toward the right hepatic vein, and the surgeon should take care not to injure the cava or the venous branches from the caudate lobe.
Pathophysiology
In general, the amount of damage caused by penetrating missiles to the liver is determined by the kinetic energy. This kinetic energy is a reflection of the mass of the missile, the differential of the velocity of the missile as it enters the tissue, and the velocity of the missile as it exits the tissue. In the 1990s, trauma centers saw an increasing number of medium-velocity injuries. Typically, these are caused by large-caliber semiautomatic and automatic weapons. More extensive injuries are seen after a shotgun blast and occasional assault rifle injuries.
Blunt trauma typically results from direct compressive forces or shear forces. The elastic tissues within arterial blood vessels make them less susceptible to tearing than any other structure within the liver. Venous and biliary ductal tissue is moderately resistant to shear forces; the liver parenchyma is the least resistant of all. Thus, fractures within the liver parenchyma tend to occur along segmental fissures or directly into the parenchyma, causing shearing of lateral branches of the major hepatic vein and portal veins. With severe deceleration injury, the origins of the hepatic veins may be ripped from the cava, causing devastating hemorrhage. Similarly, the small branches from the caudate lobe entering directly into the cava are at high risk for shear, and thus a linear tear appears on the anterior caval surface. Direct compressive forces usually cause tearing between segmental fissures in an anteroposterior sagittal orientation. Horizontal fracture lines into the parenchyma give the characteristic burst pattern to such liver injuries. These usually underlie the ribs and costal cartilage. Fracture lines that are parallel have been dubbed bear claw–type injuries. Occasionally, there will be a single fracture line across the horizontal plane of the liver, usually between the anterior and posterior segments. Because this fracture line involves both lobes of the liver, it can cause significant hemorrhage if
there is direct extension or continuity with the peritoneal cavity.
there is direct extension or continuity with the peritoneal cavity.
Knowing the mechanism of injury articulated by the paramedics allows the surgeon to anticipate certain patterns of injury. Compressive forces caused by the steering wheel or the shoulder belt of a three-point restraint system can result in extensive bear claw–type injuries to the liver and even transections of the liver (Fig. 7). Another example is the so-called T-bone auto crash, which is a crash of two vehicles in a perpendicular fashion. Extensive injury can occur to the liver, usually when the T-bone is into the passenger side, which causes extensive right lateral rib fractures and compression of the right lobe of the liver. An extreme form of this lateral compressive injury is a transverse fracture through both lobes of the liver (Fig. 8). Shear injuries are usually associated with deceleration from falls (from a distance greater than two floors) or unrestrained occupants in high-speed motor vehicle accidents. The abrupt deceleration tends to tear the “relatively” heavy liver from its attachments, such as hepatic veins, veins from the caudate lobe, and lacerations into parenchyma at the ligamentum teres, which are often associated with exsanguinating hemorrhage.
The liver is at a high risk for injury from penetrating wounds, because it is the largest parenchymatous organ and its anatomic location is at the junction of the upper one-third and middle one-third of the torso. Major hepatic trauma is usually associated with medium- and high-velocity wounds. Occasionally, major vessel injury (usually involving the hepatic vein) can be caused by stab wounds, and I have seen several made with large knives, such as bayonets or bowie-type weapons. If a patient with a penetrating wound to the torso, particularly the midportion, is in a hemodynamically unstable condition, he or she requires no diagnostic studies except a chest radiograph, which may help the surgeon decide which torso cavity to open first. If one of the hemithoraces is full of blood, as determined by chest radiograph, it may be prudent to open that cavity first. The pitfall is that a hole in the diaphragm with injury to the left or right lobe of the liver can be the source of exsanguination. This possibility makes the point that the trauma patient should never be in any other position than the supine one. The surgeon must be prepared to deal with exsanguination on both sides of the diaphragm. Rarely, a patient with major hepatic trauma can present in a hemodynamically stable condition from penetrating wounds. If there is a high index of suspicion of penetration into the abdominal cavity or if peritoneal signs are present, exploratory celiotomy is indicated. If in doubt, the surgeon can use diagnostic peritoneal lavage or ultrasound as a diagnostic adjunct to confirm that intraperitoneal blood is present. It must be emphasized that in the hemodynamically unstable patient with penetrating wounds to the torso there is no diagnostic challenge. The patient requires immediate surgery.
From a diagnostic standpoint, major hepatic injury from blunt trauma can be more vexing than trauma from penetrating wounds, particularly in the young healthy adult who can compensate for significant blood loss.
Physical examination, even in an alert patient, is accurate in only one half of cases and carries a 56% false-positive rate and a 34% to 46% false-negative rate. The introduction of diagnostic peritoneal lavage in 1965 dramatically improved the surgeon’s ability to diagnose intra-abdominal injury. It is rapidly performed and highly sensitive. More recently, the CT scan has been shown to be an even more valuable diagnostic tool, because it allows the surgeon to make judgments as to whether the patient requires operative intervention. The CT scan is valuable for even extensive liver lacerations. If these lacerations are contained and there is minimal blood into the peritoneal cavity, it may be more prudent to treat the patient nonoperatively.
Physical examination, even in an alert patient, is accurate in only one half of cases and carries a 56% false-positive rate and a 34% to 46% false-negative rate. The introduction of diagnostic peritoneal lavage in 1965 dramatically improved the surgeon’s ability to diagnose intra-abdominal injury. It is rapidly performed and highly sensitive. More recently, the CT scan has been shown to be an even more valuable diagnostic tool, because it allows the surgeon to make judgments as to whether the patient requires operative intervention. The CT scan is valuable for even extensive liver lacerations. If these lacerations are contained and there is minimal blood into the peritoneal cavity, it may be more prudent to treat the patient nonoperatively.
Ultrasound has also been advocated as a diagnostic adjunct. It has an 85% to 90% sensitivity in picking up intraperitoneal blood but it does not meet the high resolution standards of CT. Because of its relative simplicity and low cost, it has been advocated as a useful technique. Intraoperative ultrasound has been found to be useful in elective surgery for patients who require resection for hepatic tumors. The ultrasound can identify metastatic lesions, and it has been useful in mapping out the venous anatomy for living-related hepatic transplant donors. I have not found it that useful during surgery for liver trauma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree